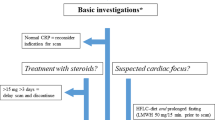
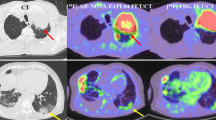
Abstract
Positron emission tomography/computed tomography (PET/CT) is a nuclear medicine functional imaging technique with proven clinical value in oncology. PET/CT indications are continually evolving with fresh advances made through research. French practice on the use of PET in oncology was framed in recommendations based on Standards–Options–Recommendations methodology and coordinated by the French federation of Comprehensive Cancer Centres (FNLCC). The recommendations were originally issued in 2002 followed by an update in 2003, but since then, a huge number of scientific papers have been published and new tracers have been licenced for market release. The aim of this work is to bring the 2003 version recommendations up to date. For this purpose, a focus group was set up in collaboration with the French Society for Nuclear Medicine (SFMN) to work on developing good clinical practice recommendations. These good clinical practice recommendations have been awarded joint French National Heath Authority (HAS) and French Cancer Institute (INCa) label status—the stamp of methodological approval. The present document is the outcome of comprehensive literature review and rigorous appraisal by a panel of experts, organ specialists, clinical oncologists, surgeons and imaging specialists. These data were also used for the EANM referral guidelines.
Similar content being viewed by others

References
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195(5):1436–43.
von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. (68)Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2018;4(5):686–93.
Bauman G, Martin P, Thiessen JD, Taylor R, Moussa M, Gaed M, et al. [(18)F]-DCFPyL positron emission tomography/magnetic resonance imaging for localization of dominant intraprostatic foci: first experience. Eur Urol Focus. 2018;4(5):702–6.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–88.
Udovicich C, Perera M, Hofman MS, Siva S, Del Rio A, Murphy DG, et al. (68)Ga-prostate-specific membrane antigen-positron emission tomography/computed tomography in advanced prostate cancer: current state and future trends. Prostate Int. 2017;5(4):125–9.
Yaxley JW, Raveenthiran S, Nouhaud FX, Samartunga H, Yaxley AJ, Coughlin G, et al. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with (68)Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology. J Urol. 2019;201(4):815–20.
Kim SJ, Lee SW, Ha HK. Diagnostic performance of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography for primary lymph node staging in newly diagnosed intermediate to high-risk prostate cancer patients: a systematic review and meta-analysis. Urol Int. 2019;102(1):27–36.
Gupta M, Choudhury PS, Rawal S, Goel HC, Singh A, Talwar V, et al. Risk stratification and staging in prostate cancer with prostatic specific membrane antigen PET/CTObjective: a one-stop-shop. Hell J Nucl Med. 2017;20(Suppl:156).
Lin CY, Lee MT, Lin CL, Kao CH. Comparing the staging/restaging performance of 68Ga-labeled prostate-specific membrane antigen and 18F-choline PET/CT in prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2019;44(5):365–76.
Dyrberg E, Hendel HW, Huynh THV, Klausen TW, Logager VB, Madsen C, et al. (68)Ga-PSMA-PET/CT in comparison with (18)F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol. 2019;29(3):1221–30.
Hegemann NS, Wenter V, Spath S, Kusumo N, Li M, Bartenstein P, et al. Distribution of prostate nodes: a PET/CT-derived anatomic atlas of prostate cancer patients before and after surgical treatment. Radiat Oncol. 2016;11:37.
Wu SY, Boreta L, Shinohara K, Nguyen H, Gottschalk AR, Hsu IC, et al. Impact of staging (68)Ga-PSMA-11 PET scans on radiation treatment plansin patients with prostate cancer. Urology. 2019;125:154–62.
Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of (68)Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2018;59(1):82–8.
Soldatov A, von Klot CAJ, Walacides D, Derlin T, Bengel FM, Ross TL, et al. Patterns of progression after (68)Ga-PSMA-ligand PET/CT-guided radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys. 2019;103(1):95–104.
De Bari B, Mazzola R, Aiello D, Aloi D, Gatta R, Corradini S, et al. ((68)Ga)-PSMA-PET/CT for the detection of postoperative prostate cancer recurrence: possible implications on treatment volumes for radiation therapy. Cancer radiotherapie : journal de la Societe francaise de radiotherapie oncologique. 2019;23(3):194–200.
Han S, Woo S, Kim YJ, Suh CH. Impact of (68)Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74(2):179–90.
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2019.
Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11–20.
Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-choline-PET/CT. Clin Nucl Med. 2016;41(7):515–21.
Pfister D, Porres D, Heidenreich A, Heidegger I, Knuechel R, Steib F, et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga-PSMA-HBED-CC than with (18)F-fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging. 2016;43(8):1410–7.
Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016;57(11):1713–9.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926–37.
Pereira Mestre R, Treglia G, Ferrari M, Pascale M, Mazzara C, Azinwi NC, et al. Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: a meta-analysis. Eur J Clin Investig. 2019;49(3):e13063.
Groheux D, Giacchetti S, Moretti J-L, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high (18)F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23.
Bos R, van Der Hoeven JJM, van Der Wall E, van Der Groep P, van Diest PJ, Comans EFI, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–87.
Humbert O, Berriolo-Riedinger A, Cochet A, Gauthier M, Charon-Barra C, Guiu S, et al. Prognostic relevance at 5 years of the early monitoring of neoadjuvant chemotherapy using (18)F-FDG PET in luminal HER2-negative breast cancer. Eur J Nucl Med Mol Imaging. 2014;41:416–27.
Acknowledgements
Reviewers
The list of reviewers is available in the thesaurus.
INCa and HAS project managers
MOROIS Sophie, Project manager in the Good Practices Department, INCa
DUPERRAY Marianne, Head of the Good Practices Department, INCa
DAHAN Muriel, Director of the Recommendations and Drugs Department, INCa
VERMEL Christine, Responsible for the quality mission and conformity of the expertise, INCa
LAURENCE Michel, Head of the Good Professional Practice Department, HAS
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publication realized at the initiative of the French Society for Nuclear Medicine (SFMN).
The full-text and roll-up summary versions are both available on the SFMN website: https://www.sfmn.org/index.php/la-societe/guides-et-recommandations/94-societe/guides-et-recommandations/355-recommandations-de-bonne-pratique-clinique-pour-l-utilisation-de-la-tep-en-cancerologie.
These good clinical practice recommendations have been awarded joint French National Heath Authority (HAS) and French Cancer Institute (INCa) label status, in recognition that they were developed in line with HAS and INCa-recommended rules, methods and procedures.
This article is part of the Topical Collection on Oncology – General
Rights and permissions
About this article
Cite this article
Salaün, PY., Abgral, R., Malard, O. et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur J Nucl Med Mol Imaging 47, 28–50 (2020). https://doi.org/10.1007/s00259-019-04553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04553-8