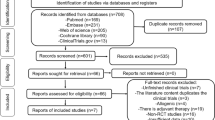
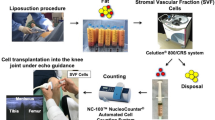
Abstract
Objective
The purpose of this study was to compare the clinical and radiological efficacy of autologous adipose-derived stromal vascular fraction (SVF) versus hyaluronic acid in patients with bilateral knee osteoarthritis.
Methods
Sixteen patients with bilateral symptomatic knee osteoarthritis (K-L grade II to III; initial pain evaluated at four or greater on a ten-point VAS score) were enrolled in this study, which were randomized into two groups. Each patient received 4-ml autologous adipose-derived SVF treatment (group test, n = 16) in one side of knee joints and a single dose of 4-ml hyaluronic acid treatment (group control, n = 16) in the other side. The clinical evaluations were performed pre-operatively and post-operatively at one month, three months, six months, and 12-months follow-up visit, using the ten-point visual analog scale (VAS), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and the knee range of motion (ROM). The whole-organ assessment of the knees was performed with whole-organ magnetic resonance imaging score (WORMS) based on MRI at baseline, six months and 12-months follow-up. The articular repair tissue was assessed quantitatively and qualitatively by magnetic resonance observation of cartilage repair tissue (MOCART) score based on follow-up MRI at six months and 12 months.
Results
No significant baseline differences were found between two groups. Safety was confirmed with no severe adverse events observed during 12-months follow-up. The SVF-treated knees showed significantly improvement in the mean VAS, WOMAC scores, and ROM at 12-months follow-up visit compared with the baseline. In contrast, the mean VAS, WOMAC scores, and ROM of the control group became even worse but not significant from baseline to the last follow-up visit. WORMS and MOCART measurements revealed a significant improvement of articular cartilage repair in SVF-treated knees compared with hyaluronic acid-treated knees.
Conclusion
The results of this study suggest that autologous adipose-derived SVF treatment is safe and can effectively relief pain, improve function, and repair cartilage defects in patients with knee osteoarthritis.



Similar content being viewed by others
References
Arden N, Nevitt MC (2006) Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 20(1):3–25. https://doi.org/10.1016/j.berh.2005.09.007
Buckwalter JA, Martin J, Mankin HJ (2000) Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect 49:481–489
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, MA AM, Memish ZA (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 380(9859):2163–2196. https://doi.org/10.1016/s0140-6736(12)61729-2
Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 (2016). Lancet (London, England) 388 (10053):1545–1602. doi:https://doi.org/10.1016/s0140-6736(16)31678-6
Leardini G, Salaffi F, Caporali R, Canesi B, Rovati L, Montanelli R (2004) Direct and indirect costs of osteoarthritis of the knee. Clin Exp Rheumatol 22(6):699–706
Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P (2007) OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthr Cartil 15(9):981–1000. https://doi.org/10.1016/j.joca.2007.06.014
Baier Leach J, Bivens KA, Patrick CW Jr, Schmidt CE (2003) Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng 82(5):578–589. https://doi.org/10.1002/bit.10605
Pagnano M, Westrich G (2005) Successful nonoperative management of chronic osteoarthritis pain of the knee: safety and efficacy of retreatment with intra-articular hyaluronans. Osteoarthr Cartil 13(9):751–761. https://doi.org/10.1016/j.joca.2005.04.012
Maricar N, Callaghan MJ, Felson DT, O’Neill TW (2013) Predictors of response to intra-articular steroid injections in knee osteoarthritis--a systematic review. Rheumatology (Oxford, England) 52(6):1022–1032. https://doi.org/10.1093/rheumatology/kes368
Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH (2016) Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 32(1):97–109. https://doi.org/10.1016/j.arthro.2015.09.010
Fodor PB, Paulseth SG (2016) Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J 36(2):229–236. https://doi.org/10.1093/asj/sjv135
Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE (2015) Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 23(5):1308–1316. https://doi.org/10.1007/s00167-013-2807-2
Koh YG, Kwon OR, Kim YS, Choi YJ (2014) Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 30(11):1453–1460. https://doi.org/10.1016/j.arthro.2014.05.036
Bansal H, Comella K, Leon J, Verma P, Agrawal D, Koka P, Ichim T (2017) Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med 15(1):141. https://doi.org/10.1186/s12967-017-1242-4
Gimble J, Guilak F (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5(5):362–369. https://doi.org/10.1080/14653240310003026
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228. https://doi.org/10.1089/107632701300062859
Spasovski D, Spasovski V, Bascarevic Z, Stojiljkovic M, Vreca M, Andelkovic M, Pavlovic S (2018) Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. The Journal of Gene Medicine 20(1). https://doi.org/10.1002/jgm.3002
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem cells (Dayton, Ohio) 32(5):1254–1266. https://doi.org/10.1002/stem.1634
Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, Yoon KS (2017) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med 45(12):2774–2783. https://doi.org/10.1177/0363546517716641
Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C (2018) Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med 13(3):295–307. https://doi.org/10.2217/rme-2017-0152
Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noel D, Canovas F, Cyteval C, Lisignoli G, Schrauth J, Haddad D, Domergue S, Noeth U, Jorgensen C (2016) Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med 5(7):847–856. https://doi.org/10.5966/sctm.2015-0245
Koh YG, Choi YJ (2012) Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 19(6):902–907. https://doi.org/10.1016/j.knee.2012.04.001
Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, Choi YJ (2013) Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 29(4):748–755. https://doi.org/10.1016/j.arthro.2012.11.017
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK (2004) Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil 12(3):177–190. https://doi.org/10.1016/j.joca.2003.11.003
Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, Trattnig S (2004) Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol 52(3):310–319. https://doi.org/10.1016/j.ejrad.2004.03.014
Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R (2016) A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopedic conditions. Int Orthod 40(8):1755–1765. https://doi.org/10.1007/s00264-016-3162-y
Vega A, Martin-Ferrero MA, Del Canto F, Alberca M, Garcia V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sanchez A, Garcia-Sancho J (2015) Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 99(8):1681–1690. https://doi.org/10.1097/tp.0000000000000678
Lisi C, Perotti C, Scudeller L, Sammarchi L, Dametti F, Musella V, Di Natali G (2018) Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil 32(3):330–339. https://doi.org/10.1177/0269215517724193
Kubosch EJ, Heidt E, Niemeyer P, Bernstein A, Sudkamp NP, Schmal H (2017) In-vitro chondrogenic potential of synovial stem cells and chondrocytes allocated for autologous chondrocyte implantation - a comparison: synovial stem cells as an alternative cell source for autologous chondrocyte implantation. Int Orthod 41(5):991–998. https://doi.org/10.1007/s00264-017-3400-y
Cuti T, Antunovic M, Marijanovic I, Ivkovic A, Vukasovic A, Matic I, Pecina M, Hudetz D (2017) Capacity of muscle derived stem cells and pericytes to promote tendon graft integration and ligamentization following anterior cruciate ligament reconstruction. Int Orthod 41(6):1189–1198. https://doi.org/10.1007/s00264-017-3437-y
Xia P, Wang X, Lin Q, Li X (2015) Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthod 39(12):2363–2372. https://doi.org/10.1007/s00264-015-2785-8
Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD (2016) Stromal vascular fraction: a regenerative reality? Part 1: current concepts and review of the literature. Journal of plastic, reconstructive & esthetic surgery: JPRAS 69(2):170–179. https://doi.org/10.1016/j.bjps.2015.10.015
Chung MT, Zimmermann AS, Paik KJ, Morrison SD, Hyun JS, Lo DD, McArdle A, Montoro DT, Walmsley GG, Senarath-Yapa K, Sorkin M, Rennert R, Chen HH, Chung AS, Vistnes D, Gurtner GC, Longaker MT, Wan DC (2013) Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl Med 2(10):808–817. https://doi.org/10.5966/sctm.2012-0183
Atalay S, Coruh A, Deniz K (2014) Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 40(7):1375–1383. https://doi.org/10.1016/j.burns.2014.01.023
Bora P, Majumdar AS (2017) Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8(1):145. https://doi.org/10.1186/s13287-017-0598-y
Riordan NH, Ichim TE, Min WP, Wang H, Solano F, Lara F, Alfaro M, Rodriguez JP, Harman RJ, Patel AN, Murphy MP, Lee RR, Minev B (2009) Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 7:29. https://doi.org/10.1186/1479-5876-7-29
You D, Jang MJ, Kim BH, Song G, Lee C, Suh N, Jeong IG, Ahn TY, Kim CS (2015) Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med 4(4):351–358. https://doi.org/10.5966/sctm.2014-0161
Funding
This study was supported by grants from National Natural Science Foundation of China (81672769) and Medical Science and Technology Foundation of Zhejiang Province (CN) (2017KY016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of Ethics Committee of the Zhejiang Provincial People’s Hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was registered at Chinses Clinical Trial Registry with identifier ChiCTR1800015125.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hong, Z., Chen, J., Zhang, S. et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. International Orthopaedics (SICOT) 43, 1123–1134 (2019). https://doi.org/10.1007/s00264-018-4099-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-4099-0