Abstract
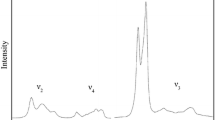
The thermal expansion of orthorhombic CaFe2O4-type β-CaCr2O4, ellinaite, was studied by high temperature in-situ synchrotron X-ray diffraction measurements in the temperature range of 301–973 K at atmospheric pressure. Based on the obtained data, the thermal expansion coefficients of β-CaCr2O4 were determined as 2.84(3) × 10–5 K−1, 1.08(1) × 10–5 K−1, 0.79(1) × 10–5 K−1, 0.99(1) × 10–5 K−1 for volume, a-, b- and c axis, respectively. An anisotropic behavior was observed because the axial expansivity for the b axis is smaller than those of the a- and c axis. Combined with available experimental results, the isobaric heat capacity (Cp) of β-CaCr2O4 was evaluated by using a Kieffer’s model with Raman spectroscopy data and compared with previous studies. The isochoric heat capacity (Cv), standard entropy (S0298) and Debye temperature (θD) of β-CaCr2O4 were also determined.





Similar content being viewed by others
References
Akaogi M, Hamada Y, Suzuki T, Kobayashi M, Okada M (1999) High pressure transitions in the system MgAl2O4-CaAl2O4: a new hexagonal aluminous phase with implications for the lower mantle. Phys Earth Planet Inter 115:67–77
Akbudak S, Candan A, Kushwaha AK, Yadav AC, Uğur G, Uğur Ş (2019) Structural, elastic, electronic and vibrational properties of XAl2O4 (X= Ca, Sr and Cd) semiconductors with orthorhombic structure. J Alloy Compd 809:51773
Albertsson GJ, Teng L, Björkman B (2014) Effect of basicity on chromium partition in CaO-MgO-SiO2-Cr2O3 synthetic slag at 1873 K. Miner Process Extr Metall 123:116–122
Anderson OL (1963) A simplified method for calculating the Debye temperature from elastic constants. J Phys Chem Solids 24:909–917
Androš L, Jurić M, Popović J, Pajić D, Zadro K, Molčanov K, Žilić D, Planinić P (2014) 1D heterometallic oxalate compounds as precursors for mixed Ca-Cr oxides—synthesis, structure, and magnetic studies. Eur J Inorg Chem 33:5703–5713
Birch F (1961) The velocity of compressional waves in rocks to 10 kilobars, Part 2. J Geophys Res 66(1961):2199–2224
Biryukov YP, Bubnova RS, Filatov SK, Goncharov AG (2016) Synthesis and thermal behavior of Fe3O2(BO4) oxoborate. Glass Phys Chem 42:202–206
Chapon LC, Manuel P, Damay F, Toledano P, Hardy V, Martin C (2011) Helical magnetic state in the distorted triangular lattice of α-CaCr2O4. Phys Rev B 83:024409
Chen M, Shu J, Mao HK, Xie X, Hemley RJ (2003) Natural occurrence and synthesis of two new post spinal polymorphs of chromite. Proc Natl Acad Sci U S A 100:14651–14654
Damay F, Martin C, Hardy V, Maignan A, Andre G, Knight K, Giblin SR, Chapon LC (2010) Zigzag ladders with staggered magnetic chirality in the S = 3/2 compound β-CaCr2O4. Phys Rev B 81:214405
Degterov S, Pelton AD (1997) Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CrO-Cr2O3-SiO2-CaO system. Metall Mater Trans B 28:235–242
Fei Y (1995) Thermal expansion. In: Ahrens TJ (ed) Mineral physics & crystallography: a handbook of physical constants. American Geophysical Union, Washington DC, pp 29–44
Funamori N, Jeanloz R, Nguyen JH, Kavner A, Cadwell WA, Fujino K, Miyajima N, Shinmei T, Tomioka N (1998) High-pressure transformation in MgAl2O4. J Geophys Res 103:20813–20818
Gao M, Gu Y, Gong Z, Li L, Gao X, Wen W (2016) Facile usage of a MYTHEN 1K with a Huber 5021 diffractometer and angular calibration in operando experiments. J Appl Crystallogr 49(2016):1182–1189
Glasser FP, Glasser LD (1962) Hexagonal high-temperature polymorph of calcium chromite. Inorg Chem 1:428–429
Hazen RM, Prewitt CT (1977) Effects of temperature and pressure on interatomic distances in oxygen-based minerals. Am Mineral 62:309–315
Hill PM, Peiser HS, Rait JR (1956) The crystal structure of calcium ferrite and β calcium chromite. Acta Crystallogr 9:981–986
Hörkner W, Müller-Buschbaum H (1976) Einkristalluntersuchungen von β-CaCr2O4. Z Naturforsch 31:1710–1711
Irifune T, Fujino K, Ohtani K (1991) A new high pressure form of MgAl2O4. Nature 349:409–411
Jacob KT, Kale GM, Abraham KP (1992) Electrochemical determination of Gibbs energies of formation of calcium chromite and chromate. J Electrochem Soc 139:517–520
Ji X, Wan W, Wang H, Li G (2011) Influence of Cr2O3 on sintering process of CaO ceramics. China Powder Sci Tech 17:54–56
Kaiser A, Sommer B, Woermann E (1992) The system CaO-CaCr2O4-CaAl2O4 in air and under mildly reducing conditions. J Am Ceram Soc 75:1463–1471
Kaminsky F, Wirth R, Schreiber A (2015) A microinclusion of lower-mantle rock and other mineral and nitrogen lower-mantle inclusions in a diamond. Can Mineral 53:83–104
Kesson SE, Fitz Gerald JD, Shelley JMG (1994) Mineral chemistry and density of subducted basaltic crust at lower-mantle pressures. Nature 372:767–769
Kieffer SW (1979a) Thermodynamics and lattice vibrations of minerals, 1. Mineral heat capacities and their relationships to simple lattice vibrational models. Rev Geophys Space Phys 17:1–19
Kieffer SW (1979b) Thermodynamics and lattice vibrations of minerals, 3. Lattice dynamics and an approximation for minerals with application to simple substances and framework silicates. Rev Geophys Space Phys 17:35–59
Kieffer SW (1980) Thermodynamics and lattice vibrations of minerals, 4. Application to chain and sheet silicates and orthosilicates. Rev Geophys Space Phys 18:862–886
Kirby SH, Stein S, Okai EA, Rubie DC (1996) Metastable mantle phase transformations and deep earthquakes in subducting oceanic lithosphere. Rev Geophys 34:261–306
Kojitani H, Nishimura K, Kubo A, Sakashita M, Aoki K, Akaogi M (2003) Raman spectroscopy and heat capacity measurement of calcium ferrite type MgAl2O4 and CaAl2O4. Phys Chem Miner 30:409–415
Kojitani H, Többens DM, Akaogi M (2013) High-pressure Raman spectroscopy, vibrational mode calculation, and heat capacity calculation of calcium ferrite-type MgAl2O4 and CaAl2O4. Am Miner 98:197–206
Kroumova E, Aroyo MI, Perez-Mato JM, Kirov A, Capillas C, Ivantchev S, Wondratschek H (2003) Bilbao crystallographic server: useful databases and tools for phase-transition studies. Phase Transit 76:155–170
Larson AC, Von Dreele RB (2000) GSAS: general structure analysis system, Los Alamos Natl Lab Rep LAUR, pp 86–748
Lee YM, Nassaralla CL (1997) Minimization of hexavalent chromium in magnesite-chrome refractory. Metall Mater Trans B 28:855–859
Lee YM, Nassaralla CL (2001) Heat capacities of calcium chromate and calcium chromite. Thermochim Acta 371:1–5
Li W, Xue X (2018) Effects of silica addition on chromium distribution in stainless-steel slag. Ironmak Steelmak 45:929–936
Liu Z, Zhang H, Luo M, Pei L, Shi Y, Cai Z, Xu H, Zhang Y (2017) Molten salt synthesis of δ-CaCr2O4 and its annealing to prepare β-CaCr2O4. Ceram Int 43:1231–1235
Luo X, Wang B (2008) Structural and elastic properties of LaAlO3 from first-principles calculations. J Appl Phys 104:073518
Merlini M, Hanfland M, Gemmi M, Huotari S, Simonelli L, Strobel P (2010) Fe3+ spin transition in CaFe2O4 at high pressure. Am Miner 95:200–203
Minh NQ (1993) Ceramic fuel cells. J Am Ceram Soc 76:563–588
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276
Morita K, Mori M, Guo M, Ikagawa T, Sano N (1999) Activity of chromium oxide and phase relations for the CaO-SiO2-CrOx system at 1873 K under moderately reducing conditions. Steel Res 70:319–324
Morita K, Tsukiashi K, Kimura M, Sano N (2005) Activity of chromium oxide in CaO-SiO2 based slags at 1873 K. Steel Res Int 76:279–283
Pillay K, Von Blottnitz H, Petersen J (2003) Ageing of chromium (III)-bearing slag and its relation to the atmospheric oxidation of solid chromium (III)-oxide in the presence of calcium oxide. Chemosphere 52:1771–1779
Rajagopalan KV, Kalyanaraman R, Sundaresan M (1989) Measurement of thermal constants of calcium chromite by laser flash method. J Therm Anal Calorim 35:1073–1077
Róg G, Kozlowska-Róg A, Dudek M (2007) The standard Gibbs free energy of formation of calcium chromium (III) oxide in the temperature range (1073 to 1273 K). J Chem Thermodyn 39:275–278
Sharygin VV (2019) Orthorhombic CaCr2O4 in phosphide-bearing gehlenite-rankinite paralava from hatrurim basin, Israel: preliminary data. Magmat Earth Relat Strateg Metal Depos 36:272
Sharygin VV, Britvin SN, Kaminsky FV, Wirth R, Nigmatulina EN, Yakovlev GA, Novoselov KA, Murashko MN (2020) Ellinaite, IMA 2019–091. CNMNC Newsletter 53, Eur J Miner 32:211
Singh K, Simon C, Toledano P (2011) Multiferroicity and magnetoelectric coupling in α-CaCr2O4. Phys Rev B 84:064129
Skinner BJ (1966) Thermal expansion. In: Clark SP (ed) Handbook of physical constants. Geological Society of America, New York, pp 75–96
Sueda Y, Irifune T, Sanehira T, Yagi T, Nishiyama N, Kikegawa T, Funakoshi K (2009) Thermal equation of state of CaFe2O4-type MgAl2O4. Phys Earth Planet Inter 174:78–85
Töth S (2012) Magnetism of 3d frustrated magnetic insulators: α-CaCr2O4, β-CaCr2O4 and Sr2VO4. Dissertation, Technische Universtität Berlin
Wulferding D, Choi KY, Lemmens P, Ponomaryov AN, Van Tol J, Islam AN, Töth S, Lake B (2012) Softened magnetic excitations in the s = 3/2 distorted triangular antiferromagnet α-CaCr2O4. J Phys Condens Matter 24:435604
Yang T, Wen W, Yin G, Li X, Gao M, Gu Y, Li L, Liu Y, Lin H, Zhang X, Zhao B, Liu T, Yang Y, Li Z, Zhou X, Gao X (2015) Introduction of the X-ray diffraction beamline of SSRF. Nucl Sci Technol 26:020101
Yin K, Zhou H, Lu X (2016) Thermodynamic properties of calcium ferrite-type MgAl2O4: a first principles study. Sci China Earth Sci 59:831–839
Zborowski J, Kronert W, Nadachouski F, Dhupia G (1987) Microstructural changes of CaO-Cr2O3 refractory under slag attack. In: Vincenzini P (ed) High Tech Ceramic. Elsevier Science, Amsterdam, p 2415
Zhai K, Xue W, Wang H, Wu X, Zhai S (2020) Raman spectra of sillimanite, andalusite, and kyanite at various temperatures. Phys Chem Miner 47:23
Zhai S, Akaogi M, Kojitani H, Xue W, Ito E (2014) Thermodynamic investigation on β- and γ-Ca3(PO4)2 and the phase equilibria. Phys Earth Planet Inter 228:144–149
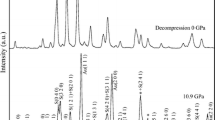
Zhai S, Yin Y, Shieh SR, Shan S, Xue W, Wang CP, Yang K, Higo Y (2016) High-pressure X-ray diffraction and Raman spectroscopy of CaFe2O4-type β-CaCr2O4. Phys Chem Miner 43:307–314
Acknowledgements
We thank Prof. Larissa Dobrzhinetskaya for the editorial handling. Critical comments and suggestion from two anonymous reviewers are helpful to improve the manuscript. The authors thank the in-situ synchrotron radiation X-ray diffraction experimental help from members at BL14B1, SSRF, China (Proposal no. 2018-SSRF-PT-007042). This work was financially supported by the National Natural Science Foundation of China (Grant No. 41872045).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, W., Zhai, K. & Zhai, S. Thermal expansion of ellinaite (β-CaCr2O4): an in-situ high temperature X-ray diffraction study. Phys Chem Minerals 48, 2 (2021). https://doi.org/10.1007/s00269-020-01126-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-020-01126-2