Abstract
Purpose
Idasanutlin, a selective small-molecule MDM2 antagonist in phase 3 testing for refractory/relapsed AML, is a non-genotoxic oral p53 activator. To optimize its dosing conditions, a number of clinical pharmacology characteristics were examined in this multi-center trial in patients with advanced solid tumors.
Method
This was an open-label, single-dose, crossover clinical pharmacology study investigating the effects of strong CYP3A4 inhibition with posaconazole (Part 1), two new oral formulations (Part 2), as well as high-energy/high-fat and low-energy/low-fat meals (Part 3) on the relative bioavailability of idasanutlin. After completing Part 1, 2, or 3, patients could have participated in an optional treatment with idasanutlin. Clinical endpoints were pharmacokinetics (PK), pharmacodynamics (PD) of MIC-1 elevation (Part 1 only), and safety/tolerability.
Results
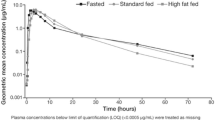
The administration of posaconazole 400 mg BID × 7 days with idasanutlin 800 mg resulted in a slight decrease (7%) in Cmax and a modest increase (31%) in AUC for idasanutlin, a marked reduction in Cmax (~ 60%) and AUC0 (~ 50%) for M4 metabolite, and a minimal increase (~ 24%) in serum MIC-1 levels. Cmax and AUC were both 45% higher for the SDP formulation. While the low-fat meal caused a less than 20% increase in all PK exposure parameters with the 90% CI values just outside the upper end of the equivalence criteria (80–125%), the high-fat meal reached bioequivalence with dosing under fasting.
Conclusion
In patients with solid tumors, multiple doses of posaconazole, a strong CYP3A4 inhibitor, minimally affected idasanutlin PK and PD without clinical significance. The SDP formulation improved rBA/exposures by ~ 50% without major food effect.




Similar content being viewed by others
References
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA (2004) In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303:844–848. https://doi.org/10.1126/science.1092472
Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, Chu XJ, Bartkovitz D, Podlaski F, Janson C, Tovar C, Filipovic ZM, Higgins B, Glenn K, Packman K, Vassilev LT, Graves B (2013) Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem 56(14):5979–5983
Higgins B, Glenn K, Walz A et al (2014) Preclinical optimization of MDM2 antagonist scheduling for cancer treatment by using a model-based approach. Clin Cancer Res 20:3742–3752
Glenn KJ, Yu LJ, Reddy MB, Fretland AJ, Parrott N, Hussain S, Palacios M, Vazvaei F, Zhi J, Tuerck D (2015) Investigating the effect of autoinduction in cynomolgus monkeys of a novel anticancer MDM2 antagonist, idasanutlin, and relevance to humans. Xenobiotica 19:1–10
Siu L, Italiano A, Miller W, Blay J, Gietema J, Bang Y, Mileshkin L, Hirte H, Reckner M, Higgins B, Jukofsky L, Blotner S, Zhi J, Middleton S, Nichols G, Chen L (2014) Phase 1 dose escalation, food effect, and biomarker study of RG7388, a more potent second-generation MDM2 antagonist, in patients (pts) with solid tumors. J Clin Oncol 2014(suppl):abstr 2535 (2014 ASCO Annual Meeting; May 30-June 3, 2014; Chicago, IL, USA. p. 5s)
Yee K, Martinelli G, Vey N, Dickinson MJ, Seiter K, Assouline S, Drummond M, Yoon S, Kasner M, Lee J, Kelly KR, Blotner S, Higgins B, Middleton S, Nichols G, Chen G, Zhong H, Pierceall WE, Zhi J, Chen L (2014) Phase 1/1b study of RG7388, a potent MDM2 antagonist, in acute myelogenous leukemia (AML) patients (Pts). Blood 124(21):116
Ananda-Rajah MR, Grigg A, Downey MT et al (2012) Comparative clinical effectiveness of prophylactic voriconazole/posaconazole to fluconazole/itraconazole in patients with acute myeloid leukemia/myelodysplastic syndrome undergoing cytotoxic chemotherapy over a 12-year period. Haematologica 97(3):459–463
Cornely OA, Maertens J, Winston DJ et al (2007) Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. New Eng J Med 356:348–359
Moton A, Ma L, Krishna G et al (2009) Effects of oral posaconazole on the pharmacokinetics of sirolimus. Curr Med Res Opin 25:701–707
Patnaik A, Tolcher A, Beeram M, Nemunaitis J, Weiss GJ, Bhalla K, Agrawal M, Nichols G, Middleton S, Beryozkina A et al (2015) Clinical pharmacology characterization of RG7112, an MDM2 antagonist, in patients with advanced solid tumors. Cancer Chemother Pharmacol 76:587–595
Acknowledgements
The authors would like to acknowledge key contributions from Roche colleagues, investigational site staff, and patient volunteers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was sponsored by F. Hoffmann-La Roche, Basel, Switzerland. AY, L-CC, GN, SB, FV, and JZ are employees of and own stock in Hoffmann-La Roche. Wilson Miller has received travel support and honoraria from Roche. JN,SE, and AR declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nemunaitis, J., Young, A., Ejadi, S. et al. Effects of posaconazole (a strong CYP3A4 inhibitor), two new tablet formulations, and food on the pharmacokinetics of idasanutlin, an MDM2 antagonist, in patients with advanced solid tumors. Cancer Chemother Pharmacol 81, 529–537 (2018). https://doi.org/10.1007/s00280-018-3521-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3521-z