Abstract
Purpose
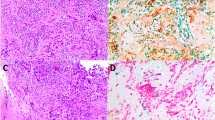
High activity of enzyme TOP2a in tumor cells is known to be associated with sensitivity to anthracycline chemotherapy, but 20% of such patients do not show clinical response. Tumor microenvironment, including tumor-associated macrophages (TAM), is an essential factor defining the efficiency of chemotherapy. In the present study, we analyzed the expression of M2 macrophage markers, YKL-39 and CCL18, in tumors of breast cancer patients received anthracycline-based NAC.
Methods
Patients were divided into two groups according to the level of doxorubicin sensitivity marker TOP2a: DOX-Sense and DOX-Res groups. Expression levels of TOR2a, CD68, YKL-39 and CCL18 genes were analyzed by qPCR, the amplification of TOR2a gene locus was assessed by the microarray assay. Clinical and pathological responses to neoadjuvant chemotherapy were assessed.
Results
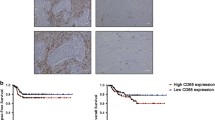
We found that the average level of TOP2a expression in patients of DOX-Sense group was almost 10 times higher than in patients of DOX-Res group, and the expression of CD68 was 3 times higher in the DOX-Sense group compared to DOX-Res group. We demonstrated that expression levels of M2-derived cytokines but not the amount of TAM is indicative for clinical and pathological chemotherapy efficacy in breast cancer patients. Out of 8 patients from DOX-Sense group who did not respond to neoadjuvant chemotherapy (NAC), 7 patients had M2+ macrophage phenotype (YKL-39+CCL18− or YKL-39−CCL18+) and only one patient had M2− macrophage phenotype (YKL-39−CCL18−). In DOX-Res group, out of 14 patients who clinically responded to NAC 9 patients had M2− phenotype and only 5 patients had M2+ macrophage phenotype. Among pathological non-responders in DOX-Sense group, 19 (82%) patients had M2+ tumor phenotype and only 4 (18%) patients had M2− phenotype. In DOX-Res group, all 5 patients who pathologically responded to NAC had M2 phenotype (YKL-39−CCL18−). Unlike the clinical response to NAC, the differences in the frequency of M2+ and M2− phenotypes between pathologically responding and non-responding patients within DOX-Sense and DOX-Res groups were statistically significant.
Conclusions
Thus, we showed that in patients with breast cancer who received anthracycline-containing NAC the absence of clinical response is associated with the presence of M2+ macrophage phenotype (YKL-39-CCL18 + or YKL-39 + CCL18-) based on TOP2a overexpression data.





Similar content being viewed by others
References
Squires H, Pandor A, Thokala P, Stevens JW, Kaltenthaler E, Clowes M, Coleman R, Wyld L (2017) Pertuzumab for the neoadjuvant treatment of early-stage HER2-positive breast cancer: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. https://doi.org/10.1007/s40273-017-0556-7
Rubovszky G, Horváth Z (2017) Recent advances in the neoadjuvant treatment of breast cancer. J Breast Cancer 20(2):119–131. https://doi.org/10.4048/jbc.2017.20.2.119
Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19(5):1508–1516. https://doi.org/10.1245/s10434-011-2108-2
El Rebey HS, Aiad HA, Abulkheir IL, Asaad NY, El-Wahed M, Abulkasem FM, Mahmoud SF (2016) The predictive and prognostic role of topoisomerase iiα and tissue inhibitor of metalloproteinases 1 expression in locally advanced breast carcinoma of Egyptian patients treated with anthracycline-based neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol 24(3):167–178. https://doi.org/10.1097/PAI.0000000000000154
Nitiss JL (2009) Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 9(5):338–350. https://doi.org/10.1038/nrc2607
Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, Schonau A, Gunnarsdóttir K, Olsen KE, Mouridsen H (2005) Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 23(30):7483–7490. https://doi.org/10.1200/JCO.2005.11.007
Munro A, Cameron D, Bartlett J (2010) Targeting anthracyclines in early breast cancer: new candidate predictive biomarkers emerge. Oncogene 29(38):5231–5240. https://doi.org/10.1038/onc.2010.286
Konecny GE, Pauletti G, Untch M, Wang HJ, Mobus V, Kuhn W, Thomssen C, Harbeck N, Wang L, Apple S, Janicke F, Slamon DJ (2010) Association between HER2, TOP2A, and response to anthracycline-based preoperative chemotherapy in high-risk primary breast cancer. Breast Cancer Res Treat 120(2):481–489. https://doi.org/10.1007/s10549-010-0744-z
Nikolényi A, Sükösd F, Kaizer L, Csörgő E, Vörös A, Uhercsák G, Ormándi K, Lázár G, Thurzó L, Brodowicz T (2011) Tumor topoisomerase II alpha status and response to anthracycline-based neoadjuvant chemotherapy in breast cancer. Oncology 80(3–4):269–277. https://doi.org/10.1159/000329038
Schindlbeck C, Janni W, Shabani N, Kornmeier A, Rack B, Rjosk D, Gerber B, Braun S, Sommer H, Friese K (2005) Isolated tumor cells in the bone marrow (ITC-BM) of breast cancer patients before and after anthracyclin based therapy: influenced by the HER2-and Topoisomerase IIα-status of the primary tumor? J Cancer Res Clin Oncol 131(8):539 – 46. https://doi.org/10.1007/s00432-005-0683-y
Kazantseva PV, Tsyganov ММ, Slonimskaya ЕМ, Litvyakov NV, Cherdyntseva NV, Ibragimova МK, Doroshenko АV, Tarabanovskaya N, Patalyak SV (2016) Molecular-genetic markers of response to neoadjuvant chemotherapy with anthracyclines in breast cancer patients. Sib J Oncol 15(2):29–35. https://doi.org/10.21294/1814-4861-2016-15-2-29-35
Arpino G, Ciocca DR, Weiss H, Allred DC, Daguerre P, Vargas-Roig L, Leuzzi M, Gago F, Elledge R, Mohsin SK (2005) Predictive value of apoptosis, proliferation, HER-2, and topoisomerase IIalpha for anthracycline chemotherapy in locally advanced breast cancer. Breast Cancer Res Treat 92(1):69–75. https://doi.org/10.1007/s10549-005-1721-9
Stakheyeva M, Riabov V, Mitrofanova I, Litviakov N, Choynzonov E, Cherdyntseva N, Kzhyshkowska J (2017) Role of the immune component of tumor microenvironment in the efficiency of cancer treatment: perspectives for the personalized therapy. Curr Pharm Des. https://doi.org/10.2174/1381612823666170714161703
Mitrofanova I, Zavyalova M, Telegina N, Buldakov M, Riabov V, Cherdyntseva N, Kzhyshkowska J (2017) Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy. Immunobiology 222(1):101–109. https://doi.org/10.1016/j.imbio.2016.08.001
Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J (2014) Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol 5:75. https://doi.org/10.3389/fphys.2014.00075
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217
Mantovani A, Polentarutti N, Luini W, Peri G, Spreafico F (1979) Role of host defense mechanisms in the antitumor activity of adriamycin and daunomycin in mice. J Natl Cancer Inst 63(1):61–66
Mantovani A, Allavena P (2015) The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 212(4):435–445. https://doi.org/10.1084/jem.20150295
Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH (2013) Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res 73(8):2480–2492. https://doi.org/10.1158/0008-5472.CAN-12-3542
Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73(3):1128–1141. https://doi.org/10.1158/0008-5472.CAN-12-2731
Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q (2014) B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 25(6):809–821. https://doi.org/10.1016/j.ccr.2014.04.026
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1(1):54–67. https://doi.org/10.1158/2159-8274.CD-10-0028
Schwartz GF, Hortobagyi GN (2004) Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26–28, 2003, Philadelphia, Pennsylvania. Cancer 100(12):2512–2532. https://doi.org/10.1002/cncr.20298
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 131(1):18–43. https://doi.org/10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12(5):320–327
Hayward JL, Carbone PP, Heuson JC, Kumaoka S, Segaloff A, Rubens RD (1977) Assessment of response to therapy in advanced breast cancer: a project of the Programme on Clinical Oncology of the International Union Against Cancer, Geneva, Switzerland. Cancer 39(3):1289–1294
Cai H, Kumar N, Baudis M (2012) arraymap: A reference resource for genomic copy number imbalances in human malignancies. PLoS One 7(5):e36944. https://doi.org/10.1371/journal.pone.0036944
Litviakov NV, Cherdyntseva NV, Tsyganov MM, Denisov EV, Garbukov EY, Merzliakova MK, Volkomorov VV, Vtorushin SV, Zavyalova MV, Slonimskaya EM (2013) Changing the expression vector of multidrug resistance genes is related to neoadjuvant chemotherapy response. Cancer Chemother Pharmacol 71(1):153–163. https://doi.org/10.1007/s00280-012-1992-x
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61. https://doi.org/10.1016/j.immuni.2014.06.010
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196(3):254–265. https://doi.org/10.1002/path.1027
De Palma M, Lewis CE (2011) Cancer: macrophages limit chemotherapy. Nature 472(7343):303–304. https://doi.org/10.1038/472303a
Hughes R, Qian B-Z, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M (2015) Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res 75(17):3479–3491. https://doi.org/10.1158/0008-5472.CAN-14-3587
Ibragimova M, Tsyganov M, Litviakov N (2017) Natural and chemotherapy-induced clonal evolution of tumors. Biochemistry 82(4):413–425. https://doi.org/10.1134/S0006297917040022
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G (2013) Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39(1):74–88. https://doi.org/10.1016/j.immuni.2013.06.014
Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N (2014) Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res 74(1):104–118. https://doi.org/10.1158/0008-5472
Bracci L, Schiavoni G, Sistigu A, Belardelli F (2014) Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 21(1):15–25. https://doi.org/10.1038/cdd.2013.67
Litviakov N, Cherdyntseva N, Tsyganov M, Slonimskaya E, Ibragimova M, Kazantseva P, Kzhyshkowska J, Choinzonov E (2016) Deletions of multidrug resistance gene loci in breast cancer leads to the down-regulation of its expression and predict tumor response to neoadjuvant chemotherapy. Oncotarget 7(7):7829–7841. https://doi.org/10.18632/oncotarget.6953
Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, Liu B, Deng H, Wang F, Lin L (2011) CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 19(4):541–555. https://doi.org/10.1016/j.ccr.2011.02.006
Lin L, Chen Y-S, Yao Y-D, Chen J-Q, Chen J-N, Huang S-Y, Zeng Y-J, Yao H-R, Zeng S-H, Fu Y-S (2015) CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget 6(33):34758–34773. https://doi.org/10.18632/oncotarget.5325
Nagarsheth N, Wicha MS, Zou W (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 17(9):559–572. https://doi.org/10.1038/nri.2017.49
Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I (2016) Role of chitinase-like proteins in cancer. Biol Chem 397(3):231–247. https://doi.org/10.1515/hsz-2015-0269
Kzhyshkowska J, Gratchev A, Goerdt S (2007) Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights 2:128–146
Shao R, Hamel K, Petersen L, Cao JQ, Arenas RB, Bigelow C, Bentley B, Yan W (2009) YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 28(50):4456–4468. https://doi.org/10.1038/onc.2009.292
Gratchev A, Kzhyshkowska J, Kannookadan S, Ochsenreiter M, Popova A, Yu X, Mamidi S, Stonehouse-Usselmann E, Muller-Molinet I, Gooi L (2008) Activation of a TGF-β-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-β receptor II. J Immunol 180(10):6553–6565. https://doi.org/10.4049/jimmunol.180.10.6553
Acknowledgements
The study was supported by the Russian Scientific Foundation, Grant #14-15-00350.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Litviakov, N., Tsyganov, M., Larionova, I. et al. Expression of M2 macrophage markers YKL-39 and CCL18 in breast cancer is associated with the effect of neoadjuvant chemotherapy. Cancer Chemother Pharmacol 82, 99–109 (2018). https://doi.org/10.1007/s00280-018-3594-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3594-8