Abstract
Purpose
To develop and validate a population pharmacokinetic model of Methotrexate (MTX) in Mexican children with acute lymphoblastic leukemia (ALL) for the design of personalized dosage regimens based on the anthropometric and physiological characteristics of each patient.
Methods
A prospective study was developed in 50 children (1–15 years old) with ALL diagnosis attended at Pediatric Hemato-Oncology Service from Hospital Central “Dr. Ignacio Morones Prieto” and under treatment with high doses of MTX administered in 24-h continuous intravenous infusion. Plasma concentrations of MTX were determined in blood samples collected at 24, 36, 42 or 48 h post-infusion, by means of the CMIA immunoassay. The development of the population pharmacokinetic model was performed using the NONMEM® software evaluating the covariates that influence in clearance (CL), intercompartmental clearance (Q), central (Vc) and peripheral (Vp) volume of distribution of MTX.
Results
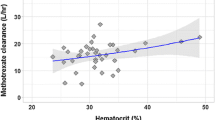
A two-compartment open model was selected to describe concentration–time data and body surface area (BSA) was the covariate that influences on MTX total CL. The population pharmacokinetic model obtained was: CL (L/h) = 6.5 × BSA0.62, Vc (L) = 0.36 × Weight, Q (L/h) = 0.41 and Vp (L) = 3.2. Internal validation was performed by bootstrap and visual predictive check. Predictive performance of final model was evaluated by external validation in a different group of patients. Initial MTX dosing regimens were established by stochastic simulation with final population pharmacokinetic model.
Conclusions
The establishment of MTX dosing criteria in children with ALL should be adjusted based on the BSA of each patient to optimize oncological therapy and reduce the development of adverse effects. Therapeutic drug monitoring is an essential tool to individualize MTX doses to reduce toxicity and improve patients’ outcomes.



Similar content being viewed by others
References
Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373(16):1541–1552. https://doi.org/10.1056/NEJMra1400972
Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, Hesseling P, Shin HY, Stiller CA, contributors I (2017) International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol 18(6):719–731. https://doi.org/10.1016/S1470-2045(17)30186-9
Castro-Rios A, Reyes-Morales H, Pelcastre-Villafuerte BE, Rendon-Macias ME, Fajardo-Gutierrez A (2019) Socioeconomic inequalities in survival of children with acute lymphoblastic leukemia insured by social security in Mexico: a study of the 2007-2009 cohorts. Int J Equity Health 18(1):40. https://doi.org/10.1186/s12939-019-0940-3
Inaba H, Greaves M, Mullighan CG (2013) Acute lymphoblastic leukaemia. Lancet 381(9881):1943–1955. https://doi.org/10.1016/S0140-6736(12)62187-4
Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, Lipshultz SE, Camitta BM (2011) Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the children’s oncology group (POG 9404). Blood 118(4):874–883. https://doi.org/10.1182/blood-2010-06-292615
Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, Mattano LA Jr, Cole C, Eicher A, Haugan M, Sorenson M, Heerema NA, Carroll AA, Gastier-Foster JM, Borowitz MJ, Wood BL, Willman CL, Winick NJ, Hunger SP, Carroll WL (2016) Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk b-acute lymphoblastic leukemia: a report from children’s oncology group study AALL0232. J Clin Oncol 34(20):2380–2388. https://doi.org/10.1200/JCO.2015.62.4544
Niemeyer CM, Gelber RD, Tarbell NJ, Donnelly M, Clavell LA, Blattner SR, Donahue K, Cohen HJ, Sallan SE (1991) Low-dose versus high-dose methotrexate during remission induction in childhood acute lymphoblastic leukemia (protocol 81-01 update). Blood 78(10):2514–2519
Evans WE, Crom WR, Abromowitch M, Dodge R, Look AT, Bowman WP, George SL, Pui CH (1986) Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med 314(8):471–477. https://doi.org/10.1056/nejm198602203140803
Evans WE, Relling MV, Boyett JM, Pui CH (1997) Does pharmacokinetic variability influence the efficacy of high-dose methotrexate for the treatment of children with acute lymphoblastic leukemia: what can we learn from small studies? Leuk Res 21(5):435–437
Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH (1998) Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med 338(8):499–505. https://doi.org/10.1056/NEJM199802193380803
Schmiegelow K (2009) Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol 146(5):489–503. https://doi.org/10.1111/j.1365-2141.2009.07765.x
Bleyer WA (1978) The clinical pharmacology of methotrexate: new applications of an old drug. Cancer 41(1):36–51
Wall AM, Gajjar A, Link A, Mahmoud H, Pui CH, Relling MV (2000) Individualized methotrexate dosing in children with relapsed acute lymphoblastic leukemia. Leukemia 14(2):221–225
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11(6):694–703. https://doi.org/10.1634/theoncologist.11-6-694
Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21(12):1471–1482. https://doi.org/10.1634/theoncologist.2015-0164
Suthandiram S, Gan GG, Zain SM, Bee PC, Lian LH, Chang KM, Ong TC, Mohamed Z (2014) Effect of polymorphisms within methotrexate pathway genes on methotrexate toxicity and plasma levels in adults with hematological malignancies. Pharmacogenomics 15(11):1479–1494. https://doi.org/10.2217/pgs.14.97
Hashiguchi M, Shimizu M, Hakamata J, Tsuru T, Tanaka T, Suzaki M, Miyawaki K, Chiyoda T, Takeuchi O, Hiratsuka J, Irie S, Maruyama J, Mochizuki M (2016) Genetic polymorphisms of enzyme proteins and transporters related to methotrexate response and pharmacokinetics in a Japanese population. J Pharm Health Care Sci 2:35. https://doi.org/10.1186/s40780-016-0069-0
Lima A, Sousa H, Monteiro J, Azevedo R, Medeiros R, Seabra V (2014) Genetic polymorphisms in low-dose methotrexate transporters: current relevance as methotrexate therapeutic outcome biomarkers. Pharmacogenomics 15(12):1611–1635. https://doi.org/10.2217/pgs.14.116
Liu SG, Gao C, Zhang RD, Zhao XX, Cui L, Li WJ, Chen ZP, Yue ZX, Zhang YY, Wu MY, Wang JX, Li ZG, Zheng HY (2017) Polymorphisms in methotrexate transporters and their relationship to plasma methotrexate levels, toxicity of high-dose methotrexate, and outcome of pediatric acute lymphoblastic leukemia. Oncotarget 8(23):37761–37772. https://doi.org/10.18632/oncotarget.17781
Radtke S, Zolk O, Renner B, Paulides M, Zimmermann M, Moricke A, Stanulla M, Schrappe M, Langer T (2013) Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood 121(26):5145–5153. https://doi.org/10.1182/blood-2013-01-480335
Zaruma-Torres F, Lares-Asseff I, Reyes-Espinoza A, Loera-Castaneda V, Chairez-Hernandez I, Sosa-Macias M, Galaviz-Hernandez C, Almanza-Reyes H (2015) Association of ABCB1, ABCC5 and xanthine oxidase genetic polymorphisms with methotrexate adverse reactions in Mexican pediatric patients with ALL. Drug Metab Personal Ther 30(3):195–201. https://doi.org/10.1515/dmpt-2015-0011
Mei L, Ontiveros EP, Griffiths EA, Thompson JE, Wang ES, Wetzler M (2015) Pharmacogenetics predictive of response and toxicity in acute lymphoblastic leukemia therapy. Blood Rev 29(4):243–249. https://doi.org/10.1016/j.blre.2015.01.001
Yanagimachi M, Naruto T, Hara T, Kikuchi M, Hara R, Miyamae T, Imagawa T, Mori M, Kaneko T, Morita S, Goto H, Yokota S (2011) Influence of polymorphisms within the methotrexate pathway genes on the toxicity and efficacy of methotrexate in patients with juvenile idiopathic arthritis. Br J Clin Pharmacol 71(2):237–243. https://doi.org/10.1111/j.1365-2125.2010.03814.x
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79(3):241–257. https://doi.org/10.1016/j.cmpb.2005.04.005
Jonsson EN, Karlsson MO (1999) Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58(1):51–64
Keizer RJ, Karlsson MO, Hooker A (2013) Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT 2:e50. https://doi.org/10.1038/psp.2013.24
Schwartz GJ, Kwong T, Erway B, Warady B, Sokoll L, Hellerstein S, Dharnidharka V, Furth S, Munoz A (2009) Validation of creatinine assays utilizing HPLC and IDMS traceable standards in sera of children. Pediatric Nephrol 24(1):113–119. https://doi.org/10.1007/s00467-008-0957-0
Mould DR, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT 2:e38. https://doi.org/10.1038/psp.2013.14
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9(4):503–512
Pui CH, Relling MV, Sandlund JT, Downing JR, Campana D, Evans WE (2004) Rationale and design of total therapy study XV for newly diagnosed childhood acute lymphoblastic leukemia. Ann Hematol 83(Suppl 1):S124–S126. https://doi.org/10.1007/s00277-004-0850-2
Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, Garcia MJ (2006) Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet 45(12):1227–1238. https://doi.org/10.2165/00003088-200645120-00007
Nader A, Zahran N, Alshammaa A, Altaweel H, Kassem N, Wilby KJ (2017) Population pharmacokinetics of intravenous methotrexate in patients with hematological malignancies: utilization of routine clinical monitoring parameters. Eur J Drug Metab Pharmacokinet 42(2):221–228. https://doi.org/10.1007/s13318-016-0338-1
Odoul F, Le Guellec C, Lamagnere JP, Breilh D, Saux MC, Paintaud G, Autret-Leca E (1999) Prediction of methotrexate elimination after high dose infusion in children with acute lymphoblastic leukaemia using a population pharmacokinetic approach. Fundam Clin Pharmacol 13(5):595–604
Hui KH, Chu HM, Fong PS, Cheng WTF, Lam TN (2019) Population pharmacokinetic study and individual dose adjustments of high-dose methotrexate in Chinese pediatric patients with acute lymphoblastic leukemia or osteosarcoma. J Clin Pharmacol 59(4):566–577. https://doi.org/10.1002/jcph.1349
Plard C, Bressolle F, Fakhoury M, Zhang D, Yacouben K, Rieutord A, Jacqz-Aigrain E (2007) A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 60(4):609–620. https://doi.org/10.1007/s00280-006-0394-3
El Desoky ES, Ghazally MH, Singh RP, Abdelhamid ON, Derendorf H (2011) Population pharmacokinetics of methotrexate in Egyptian children with lymphoblastic leukemia. Ther Drug Monit 33(4):548
Kim IW, Yun HY, Choi B, Han N, Park SY, Lee ES, Oh JM (2012) ABCB1 C3435T genetic polymorphism on population pharmacokinetics of methotrexate after hematopoietic stem cell transplantation in Korean patients: a prospective analysis. Clin Ther 34(8):1816–1826. https://doi.org/10.1016/j.clinthera.2012.06.022
Acknowledgments
The authors would like to thank for the assistance of Marlen Melendez, Mariana Morales and Malena Najera, as well as nurses, medical and technical staff from the Hemato-Oncology Pediatric Service at Hospital Central “Dr Ignacio Morones Prieto” and their contributions to the present study.
Funding
This work was supported by Joint Fund for the Promotion of Scientific and Technological Research (FOMIX) through National Council for Science and Technology and State Government from San Luis Potosí (Register FMSLP-2014-02-250277) and Sectorial Fund of Health Research and Social Security from National Council for Science and Technology (Register 272549).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Medellin-Garibay, S.E., Hernández-Villa, N., Correa-González, L.C. et al. Population pharmacokinetics of methotrexate in Mexican pediatric patients with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 85, 21–31 (2020). https://doi.org/10.1007/s00280-019-03977-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03977-1