Abstract
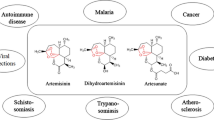
Qinghaosu, known as artemisinin (ARS), has been for over two millennia, one of the most common herbs prescribed in traditional Chinese medicine (TCM). ARS was developed as an antimalarial drug and currently belongs to the established standard treatments of malaria as a combination therapy worldwide. In addition to the antimalarial bioactivity of ARS, anticancer activities have been shown both in vitro and in vivo. Like other natural products, ARS acts in a multi-specific manner also against hematological malignancies. The chemical structure of ARS is a sesquiterpene lactone, which contains an endoperoxide bridge essential for activity. The main mechanism of action of ARS and its derivatives (artesunate, dihydroartemisinin, artemether) toward leukemia, multiple myeloma, and lymphoma cells comprises oxidative stress response, inhibition of proliferation, induction of various types of cell death as apoptosis, autophagy, ferroptosis, inhibition of angiogenesis, and signal transducers, as NF-κB, MYC, amongst others. Therefore, new pharmaceutically active compounds, dimers, trimers, and hybrid molecules, could enhance the existing therapeutic alternatives in combating hematologic malignancies. Owing to the high potency and good tolerance without side effects of ARS-type drugs, combination therapies with standard chemotherapies could be applied in the future after further clinical trials in hematological malignancies.


Similar content being viewed by others
Abbreviations
- AML:
-
Acute myeloid leukemia
- AMoL:
-
Acute monocytic leukemia
- AMPK:
-
AMP-activated protein kinase
- AP-1:
-
Activator protein-1
- Ara-C:
-
Cytarabine
- ARM:
-
Artemether
- ARS:
-
Artemisinin
- ART:
-
Artesunate
- ATO:
-
Arsenic trioxide
- B-ALL:
-
B-acute lymphoblastic leukemia
- BM:
-
Bone marrow
- CML:
-
Chronic myeloid leukemia
- CuZnSOD:
-
Copper, zinc-superoxide dismutase
- DA:
-
Decitabine
- DHA:
-
Dihydroartemisinin
- DLBCL:
-
Diffuse large B-cell lymphoma
- DM:
-
Dexamethasone
- DX:
-
Deferoxamine
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated kinase
- GEM:
-
Genetically-engineered mouse
- GpA:
-
Glycophorin A receptor
- GPX1/2:
-
Glutathione peroxidases 1 and 2
- HBO2 :
-
Hyperbaric oxygen
- HET:
-
Dihydroethidine
- H2O2 :
-
Hydrogen peroxide
- IFN-α:
-
Interferon-α
- JNK:
-
C-Jun-N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinases
- MM:
-
Multiple myeloma
- MMP:
-
Mitochondrial membrane potential
- MnSOD:
-
Manganese-superoxide dismutase
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- O2 − :
-
Superoxide
- PBMC:
-
Peripheral blood mononuclear cells
- PBN:
-
N-Tert-butyl-alpha-phenylnitrone
- P-gp:
-
P-Glycoprotein
- PPP:
-
Pentose phosphate pathway
- ROS:
-
Reactive oxygen species
- SS:
-
Sodium salicylate
- STAT3:
-
Signal transducer and activator of transcription-3
- TCM:
-
Traditional Chinese medicine
- TfR:
-
Transferrin receptor
- VEGF:
-
Vascular endothelial growth factor
- WHO:
-
World Health Organization
References
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Science 228:1049–1055
Miller LH, Su X (2011) Artemisinin: discovery from the Chinese herbal garden. Cell 146:855–858
Tu Y (2016) Artemisinin—a gift from traditional chinese medicine to the world (nobel lecture). Angew Chem. https://doi.org/10.1002/anie.201601967
Hien TT, White NJ (1993) Qinghaosu. Lancet 341:603–608
Efferth T, Dunstan F, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18:767–773
Fox JM, Moynihan JR, Mott BT, Mazzone JR, Anders NM, Brown PA, Rudek MA, Liu JO, Arav-Borger R, Posner GH, Civin CI, Chen X (2016) Artemisinin-derived dimer ART-838 potently inhibited human acute leukemias, persisted in vivo, and synergized with antileukemic drugs. Oncotarget 7:7268–7279
Zhao X, Zhong H, Wang R, Liu D, Waxman S, Zhao L, Jing Y (2015) Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget. https://doi.org/10.18632/oncotarget.3336
Kim C, Lee JH, Kim S-H, Sethi G, Ahn KS (2015) Artesunate suppresses tumor growth and induces apoptosis through the modulation of multiple oncogenic cascades in a chronic myeloid leukemia xenograft mouse model. Oncotarget. https://doi.org/10.18632/oncotarget.3004.
Lam NS, Long X, Wong JW, Griffin RC, Doery JCG (2019) Artemisinin and its derivatives: a potential treatment for leukemia. Anticancer Drugs 30:1–18
Efferth T (2017a) From ancient herb to modern drug: artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2017.02.009
Efferth T (2017b) Cancer combination therapies with artemisinin-type drugs. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2017.03.019
Kumar MS, Yadav TT, Khair RR, Peters GJ, Yergeri MC (2019) Combination therapies of artemisinin and its derivatives as a viable approach for future cancer treatment. Curr Pharm Des 25:3323–3338
Foglio MA, Dias PC, Antônio MA, Possenti A, Ferreira Rodrigues RA, Ferreira da Silva E, Rehder VL, de Carvalho JE (2002) Antiulcerogenic activity of some sesquiterpene lactones isolated from artemisia annua. Planta Med 68:515–518
Ives CS, Pedro MM, Ilza MOS, Mary AF, Eduardo L, Sérgio NE, Chagas ACS (2017) Anthelmintic activity of Artemisia annua in sheep-model. J Med Plants Res 11:137–143
Boareto AC, Muller JC, Bufalo AC, Botelho GGK, de Araujo SL, Foglio MA, de Morais RN, Dalsenter PR (2008) Toxicity of artemisinin [Artemisia annua L.] in two different periods of pregnancy in Wistar rats. Reprod Toxicol 25:239–246
Volpe Zanutto F, McAlister E, Marucci Pereira Tangerina M, Fonseca-Santos B, Costa Salles TH, Oliveira Souza IM, Brisibe A, Vilegas W, Chorilli M, d’Ávila MA, Donnelly RF, Foglio MA (2019) Semisynthetic derivative of Artemisia annua-Loaded transdermal bioadhesive for the treatment of uncomplicated malaria caused by Plasmodium falciparum in children. J Pharm Sci 108:1177–1188
Torello CO, Shiraishi RN, Della Via FI, de Castro TCL, Longhini AL, Santos I, Bombeiro AL, Araujo Silva CL, Queiroz ML, Rego EM, Saad STO (2018) Reactive oxygen species production triggers green tea-induced anti-leukaemic effects on acute promyelocytic leukaemia model. Cancer Lett 414:116–126
Alvarez MC, Maso V, Torello CO, Ferro KP, Saad STO (2018) The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin Epigenetics. https://doi.org/10.1186/s13148-018-0563-3
Bowman RL, Busque L, Levine RL (2018) Clonal Hematopoiesis and Evolution to Hematopoietic Malignancies. Cell Stem Cell 22:157–170
Kumar SK, Rajkumar V, Kyle RA, Van Duin M, Sonneveld P, Mateos MV, Gay F, Anderson KC (2017) Multiple myeloma. Nat Rev Dis Prim. https://doi.org/10.1038/nrdp.2017.46
Armitage JO, Gascoyne RD, Lunning MA, Cavalli F (2017) Non-Hodgkin lymphoma. Lancet 390:298–310
Gurnari C, Voso MT, Maciejewski JP, Visconte V (2020) From bench to bedside and beyond: therapeutic scenario in acute myeloid leukemia. Cancers (Basel) 12:1–20
De Kouchkovsky I, Abdul-Hay M (2016) Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. https://doi.org/10.1038/bcj.2016.50
Papanikolaou X, Johnson S, Garg T, Tian E, Tytarenko R, Zhang Q, Stein J, Barlogie B, Epstein J, Heuck C (2014) Artesunate overcomes drug resistance in multiple myeloma by inducing mitochondrial stress and non-caspase apoptosis. Oncotarget 5:4118–4128
Drenberg CD, Buaboonnam J, Orwick SJ, Hu S, Li L, Fan Y, Shelat AA, Guy RK, Rubnitz J, Baker SD (2016) Evaluation of artemisinins for the treatment of acute myeloid leukemia. Cancer Chemother Pharmacol 77:1231–1243
Zheng G-Q (1993) Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med. https://doi.org/10.1055/s-2006-959408
Wang Y, Li Y, Shang D, Efferth T (2019) Interactions between artemisinin derivatives and P-glycoprotein. Phytomedicine. https://doi.org/10.1016/j.phymed.2019.152998
Efferth T, Davey M, Olbrich A, Rücker G, Gebhart E, Davey R (2002) Activity of drugs from traditional Chinese medicine toward sensitive and MDR1- or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells, Mol Dis 28:160–168
Singh NP, Ferreira JFS, Park JS, Lai HC (2011) Cytotoxicity of ethanolic extracts of Artemisia annua to molt-4 human leukemia cells. Planta Med 77:1788–1793
Wickerath M, Singh NP (2014) Additive cytotoxic effects of dihydroartemisinin and sodium salicylate on cancer cells. Anticancer Res 34:3399–3401
Gerhardt T, Jones R, Park J, Lu R, Chan HW, Fang Q, Singh N, Lai H (2015) Effects of antioxidants and pro-oxidants on cytotoxicity of dihydroartemisinin to Molt-4 human leukemia cells. Anticancer Res 35:1867–1872
Kumar B, Kalvala A, Chu S, Rosen S, Forman SJ, Marcucci G, Chen CC, Pullarkat V (2017) Antileukemic activity and cellular effects of the antimalarial agent artesunate in acute myeloid leukemia. Leuk Res 59:124–135
Ohtaka M, Itoh M, Tohda S (2017) BMI1 inhibitors down-regulate NOTCH signaling and suppress proliferation of acute leukemia cells. Anticancer Res 37:6047–6053
Holien T, Olsen OE, Misund K, Hella H, Waage A, Rø TB, Sudan A (2013) Lymphoma and myeloma cells are highly sensitive to growth arrest and apoptosis induced by artesunate. Eur J Haematol 91:339–346
Mercer AE, Maggs JL, Sun XM, Cohen GM, Chadwick J, O’Neill PM, Park BK (2007) Evidence for the involvement of carbon-centered radicals in the induction of apoptotic cell death by artemisinin compounds. J Biol Chem 282:9372–9382
Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X, Ren X, An Y, Wu Y, Sun W, Fan W, Zhu Q, Wang Y, Tong X (2019) DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med 131:356–369
Lee J, Zhou HJ, Wu XH (2006) Dihydroartemisinin downregulates vascular endothelial growth factor expression and induces apoptosis in chronic myeloid leukemia K562 cells. Cancer Chemother Pharmacol 57:213–230
Li Y, Shan F, Wu JM, Wu GS, Ding J, Xiao D, Yang WY, Atassi G, Léonce S, Caignard DH, Renard P (2001) Novel antitumor artemisinin derivatives targeting G1 phase of the cell cycle. Bioorg Med Chen Lett 11:5–8
Li Y, Wu JM, Shan F, Wu GS, Ding J, Xiao D, Han JX, Atassi G, Leonce S, Caignard DH, Renard P (2003) Synthesis and cytotoxicity of dihydroartemisinin ethers containing cyanoarylmethyl group. Bioorg Med Chem 11:977–984
Wang Y, Xu X, Wu X, Chen W, Huang F, Gui X (2018) Dihydroartemisinin treatment of multiple myeloma cells causes activation of c-jun leading to cell apoptosis. Oncol Lett 15:2562–2566
Chen H, Shi L, Yang X, Li S, Guo X, Pan L (2010) Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int J Hematol 92:587–597
Wu XH, Zhou HJ, Lee J (2006) Dihydroartemisinin inhibits angiogenesis induced by multiple myeloma RPMI8226 cells under hypoxic conditions via downregulation of vascular endothelial growth factor expression and suppression of vascular endothelial growth factor secretion. Anticancer Drugs 17:839–848
Li S, Xue F, Cheng Z, Yang X, Wang S, Geng F, Pan L (2009) Effect of artesunate on inhibiting proliferation and inducing apoptosis of SP2/0 myeloma cells through affecting NFκB p65. Int J Hematol 90:513–521
Zhao X, Guo X, Yue W, Wang J, Yang J, Chen J (2017) Artemether suppresses cell proliferation and induces apoptosis in diffuse large B cell lymphoma cells. Exp Ther Med 14:4083–4090
Våtsveen TK, Myhre MR, Steen CB, Wälchli S, Lingjærde OC, Bai B, Dillard P, Theodossiou TD, Holien T, Sudan A, Inderberg EM, Smeland EB, Myklebust JH, Oksvold MP (2018) Artesunate shows potent anti-tumor activity in B-cell lymphoma. J Hematol Oncol. https://doi.org/10.1186/s13045-018-0561-0
Huo L, Wei W, Wu S, Zhao X, Zhao C, Zhao H, Sun L (2018) Effect of dihydroarteminin combined with siRNA targetingnotch1 on Notch1/c-Myc signaling in T-cell lymphoma cells. Exp Ther Med 15:3059–3065
Wei W, Zhao X, Wu S, Zhao C, Zhao H, Sun L, Cui Y (2018) Dihydroartemisinin triggers c-Myc proteolysis and inhibits protein kinase B/glycogen synthase kinase 3β pathway in T-cell lymphoma cells. Oncol Lett 16:6838–6846
Sieber S, Gdynia G, Roth W, Bona Vida B, Efferth T (2009) Combination treatment of malignant B cells using the anti-CD20 antibody rituximab and the anti-malarial artesuante. Int J Oncol 35:149–158
Ishikawa C, Senba M, Mori N (2020) Evaluation of artesunate for the treatment of adult T-cell leukemia/lymphoma. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2020.172953
Lai H, Singh NP (1995) Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett 91:41–46
Singh NP, Lai HC (2005) Synergistic cytotoxicity of artemisinin and sodium butyrate on human cancer cells. Anticancer Res 25:4325–4331
Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D, Hafer R, Stamminger T, Oesch F, Kaina B, Marschall M (2004) Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med 37:998–1009
Kelter G, Steinbach D, Konkimalla VB, Tahara T, Taketani S, Fiebig HH, Efferth T (2007) Role of transferrin receptor and the ABC transporters ABCB6 and ABCB7 for resistance and differentiation of tumor cells towards artesunate. PLoS ONE. https://doi.org/10.1371/journal.pone.0000798
Lai H, Sasaki T, Singh NP, Messay A (2005) Effects of artemisinin-tagged holotransferrin on cancer cells. Life Sci 76:1267–1279
Oh S, Kim BJ, Singh NP, Lai H, Sasaki T (2009) Synthesis and anti-cancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Lett 274:33–39
Park J, Lai HC, Singh M, Sasaki T, Singh NP (2014) Development of a dihydroartemisinin-resistant molt-4 leukemia cell line. Anticancer Res 34:2807–2810
Ohgami Y, Elstad CA, Chung E, Shirachi DY, Quock RM, Lai HC (2010) Effect of hyperbaric oxygen on the anticancer effect of artemisinin on molt-4 human leukemia cells. Anticancer Res 30:4467–4470
Wang Q, Wu S, Zhao X, Zhao C, Zhao H, Huo L (2015) Mechanisms of dihydroartemisinin and dihydroartemisinin/holotransferrin cytotoxicity in T-cell lymphoma cells. PLoS ONE 10:1–12
Lu JJ, Meng LH, Cai YJ, Chen Q, Tong LJ, Lin LP, Ding J (2008) Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogen-activated protein kinase activation but independent of reactive oxygen species. Cancer Biol Ther 7:1017–1023
Zhou H-J, Wang Z, Li A (2007) Dihydroartemisinin induces apoptosis in human leukemia cells HL60 via downregulation of transferrin receptor expression. Anti Cancer Drugs. https://doi.org/10.1097/CAD.0b013e3282f3f152.
Singh NP, Lai HC (2004) Artemisinin induces apoptosis in human cancer cells. Anticancer Res 24:2277–2280
Wang Z, Hu W, Zhang JL, Wu XH, Zhou HJ (2012) Dihydroartemisinin induces autophagy and inhibits the growth of iron-loaded human myeloid leukemia K562 cells via ROS toxicity. FEBS Open Biol 2:103–112
Efferth T, Glaisi M, Merling A, Krammer PH, Li-Weber M (2007) Artesunate induces ROS-mediated apoptosis in Doxorubicin-resistant T leukemia cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0000693
Zhang S, Chen H, Gerhard GS (2010) Heme synthesis increases artemisinin-induced radical formation and cytotoxicity that can be suppressed by superoxide scavengers. Chem Biol Interact 186:30–35
Chan HW, Singh NP, Lai HC (2013) Cytotoxicity of dihydroartemisinin toward Molt-4 cells attenuated by N-tert-butyl-alpha-phenylnitrone and deferoxamine. Anticancer Res 33:4389–4394
Wang N, Zeng GZ, Yin JL, Bian ZX (2019) Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt’s lymphoma. Biochem Biophys Res Commun 519:533–539
Gao N, Budhraja A, Cheng S, Liu EH, Huang C, Chen J, Yang Z, Chen D, Zhang Z, Shi X (2011) Interruption of the MEK/ERK signaling cascade promotes dihydroartemisinin-induced apoptosis in vitro and in vivo. Apoptosis 16:511–523
Lee J, Zhang G, Wu X, Xu F, Zhou J, Zhang X (2012) Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+chronic myeloid leukemia K562 cells involve AKT, ERK and NF-κB modulation. J Cancer Res Clin Oncol 138:2095–2102
Lee J, Shen P, Zhang G, Wu X, Zhang X (2013) Dihydroartemisinin inhibits the Bcr/Abl oncogene at the mRNA level in chronic myeloid leukemia sensitive or resistant to imatinib. Biomed Pharmacother 67:157–163
Budhraja A, Turnis ME, Churchman ML, Kothari A, Yang X, Xu H, Kaminska E, Panetta JC, Finkelstein D, Mullighan CG, Opferman JT (2017) Modulation of navitoclax sensitivity by dihydroartemisinin-mediated MCL-1 repression in BCR-ABL+ B-lineage acute lymphoblastic leukemia. Clin Cancer Res 23:7558–7568
Elf S, Lin R, Xia S, Pan Y, Shan C, Wu S, Lonial S, Gaddh M, Arellano ML, Khoury HJ, Khuri FR, Lee BH, Boggon TJ, Fan J, Chen J (2017) Targeting 6-phosphogluconate dehydrogenase in the oxidative PPP sensitizes leukemia cells to antimalarial agent dihydroartemisinin. Oncogene 36:254–262
Li Y, Feng L, Jiang W, Shan N, Wang X (2014) Artesunate possesses anti-leukemia properties that can be enhanced by arsenic trioxide. Leuk Lymphoma 55:1366–1372
Tan M, Rong Y, Su Q, Chen Y (2017) Artesunate induces apoptosis via inhibition of STAT3 in THP-1 cells. Leuk Res 62:98–103
Cao JT, Mo HM, Wang Y, Zhao K, Zhang TT, Wang CQ, Xu KL, Han ZH (2018) Dihydroartemisinin-induced apoptosis in human acute monocytic leukemia cells. Oncol Lett 15:3178–3184
Handrick R, Ontikatze T, Bauer KD, Freier F, Rübel A, Dürig J, Belka C, Jendrossek V (2010) Dihydroartemisinin induces apoptosis by a bak-dependent intrinsic pathway. Mol Cancer Ther 9:2497–2510
Sun X, Yan P, Zou C, Wong YK, Shu Y, Lee YM, Zhang C, Yang ND, Wang J, Zhang J (2019) Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Med Res Rev 39:2172–2193
Cheng C, Wang T, Song Z, Peng L, Gao M, Hermine O, Rousseaux S, Khochbin S, Mi JQ, Wang J (2018) Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044. Cancer Med 7:380–396
Hu W, Chen SS, Zhang JL, Lou XE, Zhou HJ (2014) Dihydroartemisinin induces autophagy by suppressing NF-κB activation. Cancer Lett 343:239–248
Roh JL, Kim EH, Jang H, Shin D (2017) Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol 11:254–262
Wang K, Zhang Z, Wang M, Cao X, Qi J, Wang D, Gong A, Zhu H (2019) Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des Dev Ther 13:2135–2144
Wei T, Liu J (2017) Anti-angiogenic properties of artemisinin derivatives. Int J Mol Med 40:972–978
Zhou HJ, Wang WQ, Wu GD, Lee J, Li A (2007) Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul Pharmacol 47:131–138
Kim SH, Kim HJ, Kim TS (2003) Differential involvement of protein kinase C in human promyelocytic leukemia cell differentiation enhanced by artemisinin. Eur J Pharmacol 482:67–76
Kim SH, Chun SY, Kim TS (2008) Interferon-α enhances artemisinin-induced differentiation of HL-60 leukemia cells via a PKCα/ERK pathway. Eur J Pharmacol 587:65–72
Finaurini S, Basilico N, Corbett Y, D’Alessandro S, Parapini S, Olliaro P, Haynes RK, Taramelli D (2012) Dihydroartemisinin inhibits the human erythroid cell differentiation by altering the cell cycle. Toxicology 300:57–66
Lu X, Efferth T (2020) Repurposing of artemisinin-type drugs for the treatment of acute leukemia. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.05.016
Horwedel C, Tsogoeva SB, Wei S, Efferth T (2010) Cytotoxicity of artesunic acid homo- and heterodimer molecules toward sensitive and multidrug-resistant CCRF-CEM leukemia cells. J Med Chem 53:4842–4848
Yang X, Wang W, Tan J, Song D, Li M, Liu D, Jing Y, Zhao L (2009) Synthesis of a series of novel dihydroartemisinin derivatives containing a substituted chalcone with greater cytotoxic effects in leukemia cells. Bioorg Med Chem Lett 19:4385–4388
Gaur R, Pathania AS, Malik FA, Bhakuni RS, Verma RK (2016) Synthesis of a series of novel dihydroartemisinin monomers and dimers containing chalcone as a linker and their anticancer activity. Eur J Med Chem 122:232–246
Chadwick J, Jones M, Mercer AE, Stocks PA, Ward SA, Park BK, O’Neill PM (2010) Design, synthesis and antimalarial/anticancer evaluation of spermidine linked artemisinin conjugates designed to exploit polyamine transporters in Plasmodium falciparum and HL-60 cancer cell lines. Bioorg Med Chem 18:2586–2597
Mott BT, He R, Chen X, Fox JM, Civin CI, Arav-Boger R, Posner GH (2013) Artemisinin-derived dimer phosphate esters as potent anti-cytomegalovirus (anti-CMV) and anti-cancer agents: A structure-activity study. Bioorg Med Chem 21:3702–3707
Reiter C, Fröhlich T, Gruber L, Hutterer C, Marschall M, Voigtländer C, Friedrich O, Kappes O, Efferth T, Tsogoeva, (2015) Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg Med Chem 23:5452–5458
Gruber L, Abdelfatah S, Frohlich T, Reiter C, Klein V, Tsogoeva SB, Efferth T (2018) Treatment of multidrug-resistant leukemia cells by novel artemisinin-, egonol-, and thymoquinone-derived hybrid compounds. Molecules. https://doi.org/10.3390/molecules23040841
Reiter C, Herrmann A, Çapci A, Efferth T, Tsogoeva SB (2012) New artesunic acid homodimers: Potent reversal agents of multidrug resistance in leukemia cells. Bioorg Med Chem 20:5637–5641
Letis AS, Seo EJ, Nikolaropoulos SS, Efferth T, Giannis A, Fousteris MA (2017) Synthesis and cytotoxic activity of new artemisinin hybrid molecules against human leukemia cells. Bioorg Med Chem 25:3357–3367
Reiter C, Çapci Karagöz A, Fröhlich T, Klein V, Zeino M, Viertel K, Held J, Mordmuller OSE, Anil H, Efferth T, Tsoegoeva SB (2014) Synthesis and study of cytotoxic activity of 1,2,4-trioxane- and egonol-derived hybrid molecules against Plasmodium falciparum and multidrug-resistant human leukemia cells. Eur J Med Chem 75:403–412
Reiter C, Fröhlich T, Zeino M, Marschall M, Bahsi H, Leidenberger M, Friedrich O, Kappes B, Hampel F, Efferth T, Tsogoeva SB (2015) New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur J Med Chem 97:164–172
Ha VT, Kien VT, Binh LH, Tien VD, My NTT, Nam NH, Baltas M, Hahn H, Han BW, Thao DT, Vu TK (2016) Design, synthesis and biological evaluation of novel hydroxamic acids bearing artemisinin skeleton. Bioorg Chem 1(66):63–71. https://doi.org/10.1016/j.bioorg.2016.03.008
Wu Y, Parapini S, Williams ID, Misiano P, Wong HN, Taramelli D, Basilico N, Haynes RK (2018) Facile preparation of N-glycosylated 10-piperazinyl artemisinin derivatives and evaluation of their antimalarial and cytotoxic Activities. Molecules. https://doi.org/10.3390/molecules23040841
Fröhlich T, Reiter C, Ibrahim MM, Beutel J, Hutterer C, Zeitträger I, Bahsi H, Leidenberger M, Friedrich O, Kappes B, Efferth T, Marschall M, Tsogoeva SB (2017) Synthesis of novel hybrids of quinazoline and artemisinin with high activities against plasmodium falciparum, human cytomegalovirus, and leukemia cells. ACS Omega 2:2422–2431
Fröhlich T, Reiter C, Saeed MEM, Hutterer C, Hahn F, Leidenberger M, Friedrich O, Kappes B, Marschall M, Efferth T, Tsogoeva SB (2018) Synthesis of thymoquinone-artemisinin hybrids: new potent antileukemia, antiviral, and antimalarial agents. ACS Med Chem Lett 9:534–539
Çapcı Karagöz A, Reiter C, Seo EJ, Gruber L, Hahn F, Leidenberger M, Klein V, Hampel F, Friedrich O, Marschall M, Kappes B, Efferth T, Tsogoeva SB (2018) Access to new highly potent antileukemia, antiviral and antimalarial agents via hybridization of natural products (homo)egonol, thymoquinone and artemisinin. Bioorg Med Chem 26:3610–3618
Posner GH, Cumming JN, Ploypradith P, Oh CH (1995) Evidence for Fe(IV)=O in the molecular mechanism of action of the trioxane antimalarial artemisinin. J Am Chem Soc 117:5885–5886
Eckstein-Ludwig U, Webb RJ, van Goethem DA, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S (2003) Artemisinin target the SERCA of Plasmodium falciparum. Nature 424:957–961
Bridgford JL, Xie SC, Cobbold SA, Pasaje CFA, Herrmann S, Yang T, Gillett DL, Dick LR, Ralph SA, Dogovski C, Spillman NJ, Tilley L (2018) Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat Commun. https://doi.org/10.1038/s41467-018-06221-1
Acknowledgements
The authors would like to thank São Paulo Research Foundation (FAPESP) and Ministry of Science, Technology, Innovations and Communications (CNPq) for financial support and Raquel S. Foglio for the writing assistance and English revision.
Funding
This study was funded by grant # 2017/21801-2 (STOS), 2014/16008-3 (MAF), 2016/18384-8 (MAF), São Paulo Research Foundation (FAPESP) and Ministry of Science, Technology, Innovations and Communications (CNPq) grant # 301676/2013-5 (STOS) CNPq #142286/2017 (RIM) and 401904/2012-1 (MAF).
Author information
Authors and Affiliations
Contributions
Design of the Review: RIM and STOS; Bibliographical review: RIM, STOS, MAF; Manuscript preparation: all authors. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mancuso, R.I., Foglio, M.A. & Olalla Saad, S.T. Artemisinin-type drugs for the treatment of hematological malignancies. Cancer Chemother Pharmacol 87, 1–22 (2021). https://doi.org/10.1007/s00280-020-04170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04170-5