Abstract
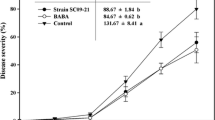
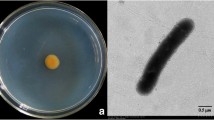
Several rhizobacteria play a vital role in plant protection, plant growth promotion and the improvement of soil health. In this study, we have isolated a strain of Lysobacter antibioticus HS124 from rhizosphere and demonstrate its antifungal activity against various pathogens including Phytophthora capsici, a destructive pathogen of pepper plants. L. antibioticus HS124 produced lytic enzymes such as chitinase, β-1,3-glucanase, lipase, protease, and an antibiotic compound. This antibiotic compound was purified by diaion HP-20, silica gel, sephadex LH-20 column chromatography and high performance liquid chromatography. The purified compound was identified as 4-hydroxyphenylacetic acid by gas chromatography-electron ionization (GC-EI) and gas chromatography-chemical ionization (GC-CI) mass spectrometry. This antibiotic exhibited destructive activity toward P. capsici hyphae. In vivo experiments utilizing green house grown pepper plants demonstrated the protective effect of L. antibioticus HS124 against P. capsici. The growth of pepper plants treated with L. antibioticus culture was enhanced, resulting in greater protection from fungal disease. Optimum growth and protection was found when cultures were grown in presence of Fe(III). Additionally, the activities of pathogenesis-related proteins such as chitinase and β-1,3-glucanase decreased in roots, but increased in leaves with time after treatment compared to controls. Our results demonstrate L. antibioticus HS124 as a promising candidate for biocontrol of P. capsici in pepper plants.






Similar content being viewed by others
References
Arthurs S, McKenzie CL, Chen J, Dogramaci M, Brennan M, Houben K, Osborne L (2009) Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera:Thripidae) on pepper. Biol Control 49:91–96
Herman MAB, Nault BA, Smart CD (2008) Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot 27:996–1002
Christensen P, Cook FD (1978) Lysobacter, a new genus of nonhiting, gliding bacteria with a high base ratio. Int J Syst Bacteriol 28:367–393
Islam MT, Hashidoko Y, Deora A, Ito T, Tahara S (2005) Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne peronosporomycetes. Appl Environ Microbiol 71:3786–3796
Mao S, Lee SJ, Hwangbo H, Kim YW, Park KH, Cha GS, Park RD, Kim KY (2006) Isolation and characterization of antifungal substances from Burkholderia sp. culture broth. Curr Microbiol 53:358–364
Meyer GD, Capieau K, Audenaert K, Buchala A, Métraux JP, Höfte M (1999) Nanogram amounts of salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 activate the systemic acquired resistance pathway in bean. Mol Plant Microbe Interact 12:450–458
Dumas-Gaudot E (1996) Plant hydrolytic enzymes (chitinases and β-l, 3-glucanases) in root reactions to pathogenic and symbiotic microorganisms. Plant Soil 185:211–221
Datta K, Tu JM, Oliva N, Ona I, Velazhahan R (2001) Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci 160:405–414
Wrobel-Kwiatkowska M, Lorenc-Kukula K, Starzycki M, Oszmianski J, Kepczynska E (2004) Expression of β-1, 3-glucanase in flax causes increased resistance to fungi. Physiol Mol Plant Pathol 65:245–256
Van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Christensen P (1989) Order II. Lysobacterales Christensen and Cook 1978, 372. 2082–2089. In: Staley JT, Bryant MP, Pfennig N, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 3. Williams and Wilkins, Baltimore
Lingappa Y, Lockwood JL (1962) Chitin media for selected isolation and culture of actinomycetes. Phytopathology 52:317–323
Yedidia I, Benhamou N, Kapulnik Y, Chet I (2000) Induction and accumulation of PR proteins activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol Biochem 38:863–873
Folman LB, Postma J, Van Veen JA (2003) Characterization of Lysobacter enzymogenes (Chtistensen and Cook 1978) strain 3.1T8, a powerful antagonist of fungal diseases of cucumber. Microbiol Res 158:107–115
Kim KD, Nemec S, Musson G (1997) Control of phytophthora root and crown rot of bell pepper with composts and soil amendments in the greenhouse. Appl Soil Ecol 5:169–179
Knievel DP (1973) Procedure for estimating ratio of live to dead root dry matter in root core samples. Crop Sci 13:124–126
Tabatabai MA (1982) Soil enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analyses, Part 2, chemical and microbiological properties, 2nd edn. American Society of Agronomy, Madison, WI, USA, pp 903–947
Folman LB, Klein M, Postma J, Van Veen JA (2004) Production of antifungal compounds by Lysobacter enzymogenes isolate 3.1T8 under different conditions in relation to its efficacy as a biocontrol agent of Pythium aphanidermatum in cucumber. Biol Control 31:145–154
Palumbo JD, Yuen GY, Jochum CC, Tatum T, Kobayashi DY (2005) Mutagenesis of β-1, 3-glucanase genes in Lysobacter enzymogenes strain C3 results in reduced biological control activity toward bipolaris leaf spot of tall fescue and pythium damping-off of sugar beet. Phytopathology 95:701–707
Russell AD, Furr JR (1996) Biocides; mechanisms of antifungal action and fungal resistance. Sci Prog 79:27–48
Stepnaya OA, Tsfasman IM, Chaika IA, Muranova TA, Kulaev IS (2008) Extracellular yeast-lytic enzyme of the bacterium Lysobacter sp. XL 1. Biochemistry 73:310–314
Zheng HZ, Kim YW, Lee HJ, Park RD, Jung WJ, Kim YC, Lee SH, Kim TH, Kim KY (2004) Quantitative changes of PR proteins and antioxidative enzymes in response to Glomus intraradices and Phytophthora capsici in pepper (Capsicum annuum L.) plants. J Microbiol Biotechnol 14:553–562
Fogliano V, Ballio A, Gallo M, Woo S, Scala F, Lorito M (2002) Pseudomonas lipodepsipeptides and fungal cell wall-degrading enzymes act synergistically in biological control. Mol Plant Microbe Interact 15:323–333
Shen SS, Choi OH, Lee SM, Park CS (2002) In vitro and in vivo activities of a biocontrol agent, Serratia plymuthica A21–4, against Phytophthora capsici. Korean J Plant Pathol 18:221–224
Lee HJ, Park KH, Shim JH, Park RD, Kim YW, Hwangbo H, Cho JY, Kim KY (2005) Isolation and identification of low molecular weight compounds produced by Bacillus subtilis HJ927 and their biocontrol effect on the late blight of pepper (Capsicum annuum L.). Korean J Soil Sci Fertil 38:25–31
Ko HS (2009) Biocontrol of phytophthora blight (Phytophthora capsici) in pepper by Lysobacter antibioticus HS124. A Master’s thesis, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, Republic of Korea
Ongenta M, Jourdan E, Adam A, Schäfer M, Budzikiewicz H, Thonart P (2007) Amino acids, iron, and growth rate as key factors influencing production of the Pseudomonas putida BTP1 benzylamine derivative involved in systemic resistance induction in different plants. Microb Ecol 55:280–292
Ryals J, Uknes S, Ward E (1994) Systemic acquired resistance. Plant Physiol 104:1109–1112
Jung WJ, Jin YL, Kim KY, Park RD, Kim TH (2005) Changes in pathogenesis-related proteins in pepper plants with regard to biological control of phytophthora blight with Paenibacillus illinoisensis. Biocontrol 50:165–178
Dassi B, Dumas-Gaudot E, Gianinazzi S (1998) Do pathogenesis-related (PR) proteins play a role in bioprotection of mycorrhizal tomato roots towards Phytophthra parasitica? Physiol Mol Plant Pathol 52:167–183
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ko, HS., Jin, RD., Krishnan, H.B. et al. Biocontrol Ability of Lysobacter antibioticus HS124 Against Phytophthora Blight Is Mediated by the Production of 4-Hydroxyphenylacetic Acid and Several Lytic Enzymes. Curr Microbiol 59, 608–615 (2009). https://doi.org/10.1007/s00284-009-9481-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9481-0