Abstract
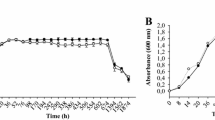
Many bacteria use small diffusible signaling molecules to communicate each other termed as quorum sensing (QS). Most Gram-negative bacteria use acyl homoserine lactone (AHL) as QS signal molecules. Using these signaling molecules, bacteria are able to express specific genes in response to population density. This work aimed to detect the production of QS signal molecules and biofilm formation in Ralstonia solanacearum isolated from various diseased tomato plants with symptoms of bacterial wilt. A total of 30 R. solanacearum strains were investigated for the production of QS signal molecules using Chromobacterium violaceum CV026 and Agrobacterium tumefaciens NT1 (pZLR4) biosensor systems. All 30 bacterial isolates from various bacterial wilt-affected tomato plants produced AHL molecules that induced the biosensor. The microtiter plate assay demonstrated that of the 30 bacterial isolates, 60 % formed biofilm, among which four isolates exhibited a higher degree of biofilm formation. The biofilm-inducing factor was purified from these four culture supernatants. The structure of the responsible molecule was solved using nuclear magnetic resonance and mass spectroscopy and was determined to be 2-hydroxy-4-((methylamino)(phenyl)methyl) cyclopentanone (HMCP), which was confirmed by chemical synthesis and NMR. The Confocal laser scanning microscopic analysis showed well-developed biofilm architecture of bacteria when treated with HMCP. The knowledge we obtained from this study will be useful for further researcher on the role of HMCP molecule in biofilm formation.







Similar content being viewed by others
References
Álvarez B, Biosca EG, López MM (2010) On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. In: Mendez-Vilas A (ed), Current research, technology and education topics in applied microbiology and microbial biotechnology, pp 267–279
Anbazhagan D, Mansor M, Yan GO, Md Yusof MY, Hassan H (2012) Detection of quorum sensing signal molecules and identification of an autoinducer synthase gene among biofilm forming clinical isolates of Acinetobacter spp. PLoS One 7:e36696
Annous BA, Fratamico PM, Smith JL (2009) Quorum sensing in biofilms: why bacteria behave the way they do. J Food Sci 74:24–37
Camilli A, Bassler BL (2006) Bacterial small-molecule signalling pathways. Science 311:1113–1116
Cataldi TR, Bianco G, Abate S (2009) Accurate mass analysis of N-acyl-homoserine-lactones and cognate lactone-opened compounds in bacterial isolates of Pseudomonas aeruginosa PAO1 by LC-ESI-LTQ-FTICR-MS. J Mass Spectrom 44:182–192
Cha C, Gao P, Chen YC, Shaw PD, Farrand SK (1998) Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol Plant-Microbe Interact 11:1119–1129
Chua SL, Hultqvist LD, Yuan M, Rybtke M, Nielsen TE, Givskov M, Tolker-Nielsen T, Yang L (2015) In vitro and in vivo generation and characterization of Pseudomonas aeruginosa biofilm-dispersed cells via c-di-GMP manipulation. Nat Protoc 10(8):1165–1180
Decho AW, Frey RL, Ferry JL (2011) Chemical challenges to bacterial AHL signaling in the environment. Chem Rev 111:86–99
Domka J, Lee J, Bansal T, Wood TK (2007) Temporal gene expression in E. Coli K-12 biofilms. Environ Microbiol 9:332–346
Farrand SK, Qin Y, Oger P (2002) Quorum-sensing system of Agrobacterium plasmids: analysis and utility. Methods Enzymol 358:452–484
Flavier AB, Clough SJ, Schell MA, Deenny TP (1997) Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol Microbiol 26(2):251–259
Garcia-Aljaro C, Vargas-Cespedes GJ, Blanch AR (2011) Detection of acylated homoserine lactones produced by Vibrio spp. and related species isolated from water and aquatic organisms. J Appl Microbiol 112:383–389
Geisenberger O, Givskov M, Riedel K, Højby N, Tu¨mmler B (2000) Production of N-acyl-l-homoserine lactones by P. aeruginosa isolates isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol Lett 184:273–278
Genin S, Denny TP (2012) Pathogenomics of the Ralstonia solanacearum species complex. Annu Rev Phytopathol 50:67–89
Kelman A (1954) The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathol 44:693–695
Lemessa F, Zeller W (2007) Isolation and characterisation of Ralstonia solanacearum strains from Solanaceae crops in Ethiopia. J Basic Microbiol 47:40–49
Loehfelm TW, Luke NR, Campagnari AA (2008) Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol 190:1036–1044
McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR (1997) Quorum sensing and Chromobacterium Õiolaceum: exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones. Microbiology 143:3703–3711
McLean RJC, Whiteley M, Stickler DJ, Fuqua WC (1997) Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol Lett 154:259–263
Morris CE, Monier JM (2003) The ecological significance of biofilm formation by plant-associated bacteria. Annu Rev Phytopathol 41:429–453
Opina N, Tavner F, Holloway G, Wang JF, Li TH (1997) A novel method for development of species and strain-specific DNA probes and PCR primers for identifying Burkholderia solanacearum (formerly Pseudomonas solanacearum). Ass Pac J Mol Biol Biotechnol 5:19–33
Pinto UM, Viana ES, Martins ML, Vanetti MCD (2007) Detection of acylated homoserine lactones in gram-negative proteolytic psychrotrophic bacteria isolated from cooled raw milk. Food Control 18:1322–1327
Plener L, Boistard P, Gonzalez A, Boucher C, Genin S (2012) Metabolic adaptation of Ralstonia solanacearum during plant infection: a methionine biosynthesis case study. PLoS One 5:e36877
Ravn L, Christensen AB, Molin S, Givskov M, Gram L (2001) Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics. J Microbiol Methods 44:239–251
Shaw PD, Ping G, Daly SL, Cha C, Cronan JE (1997) Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA 94:6036–6041
Valls M, Genin S, Boucher C (2006) Integrated regulation of the type III secretion system and other virulence determinants in Ralstonia solanacearum. PLoS Pathog 2:798–807
Xu K, Li S, Yang W, Li K, Bai Y, Xu Y, Jin J, Wang Y, Bartlam M (2015) Structural and biochemical analysis of tyrosine phosphatase related to biofilm formation A (TpbA) from the opportunistic pathogen Pseudomonas aeruginosa PAO1. PLoS One 10(4):e0124330
Zhang G, Zhang F, Ding G, Li J, Guo X (2012) Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. ISME J 6:1336–1344
Acknowledgments
The authors thank The Chairman, Department of Studies in Biotechnology, University of Mysore, Karnataka, India for supporting this work. We acknowledge the recognition of University of Mysore as an Institution of Excellence by the Government of India with financial support from the Ministry of Human Resource Development and University Grants Commission, India. We also thank Dr. Vittorio Venturi from the Bacteriology Group, International Centre for Genetic Engineering and Biotechnology, Trieste, Italy for the donation of strains C. violaceum CV026 and A. tumefaciens NT1 (pZLR4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumar, J.S., Umesha, S., Prasad, K.S. et al. Detection of Quorum Sensing Molecules and Biofilm Formation in Ralstonia solanacearum . Curr Microbiol 72, 297–305 (2016). https://doi.org/10.1007/s00284-015-0953-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-015-0953-0