Abstract
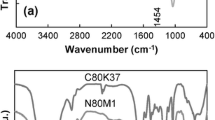
Water-soluble copolymers of acrylamide (AM) and (3-Acrylamidopropyl) trimethyl ammonium chloride (APTAC) and Na-montmorillonite (Na-MMT) were prepared by controlled radical polymerization of AM, APTAC, and Na-MMT clay using potassium persulfate (KPS) and sodium metabisulfite (MBA) as redox initiator and cobalt acetyl acetone (Co(acac)2) as a catalyst in an aqueous solution. The synthesized PAAM copolymers were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), and differential scanning calorimetry (DSC). XRD and FTIR indicated the formation of APTAC/PAAM/Co(acac)2 and PAAM/Na-MMT Co(acac)2 copolymers and also showed that the interplanar spacing increased due to intercalation formation between the clay layers. DSC indicated an increase in the thermal stability of the mentioned hydrogels, and some increases the glass transition temperature of the PAAM hydrogel in the presence of the nanoclay. The Co(acac)2 controlled the chain polymerization via a reversible activation–deactivation of the chains, so the storage modulus of the hydrogel increases significantly. Hydrogels with acceptable gel strength, gelation time, and gel stability were prepared by cross-linking of the aqueous solutions of the synthesized nanocomposite copolymer with Chromium (III) acetate for use in water shutoff operations in oil reservoirs. The effects of pH, salinity, Co(acac)2, temperature, and the presence of clay minerals on the gelation time were investigated, and the activation energies were measured. With increasing temperature, gelation occurred more rapidly. Addition of Co(acac)2 increased the loss and storage modulus, because of reversible activation–deactivation radical polymerization and decreased the gel swelling. The polymer chains diffused between the clay layers so the elastic modulus (G′) of the prepared hydrogels increased, and a reversible interaction between clay and acrylamide chains led to increase in loss modulus (G′′).












Similar content being viewed by others
References
Vargas-Vasquez SM, Romero-Zerón LB (2008) A review of the partly hydrolyzed polyacrylamide Cr(III) acetate polymer gels. Pet Sci Technol 26:481–498
El-Karsani KSM, Al-Muntasheri GA, Hussein IA (2014) Polymer systems for water shutoff and modification: a review over the last decade. SPE J 19:135–149
Arjmand O, Ahmadi M, Hosseini L (2013) An overview of the polymer gel technique to improve the efficiency of water flooding into oil reservoirs (with introduction of a new polymer). Int JChem Pet Sci 2:1–9
Semsarzadeh MA, Amiri S (2012) Controlled free radical polymerization of vinyl acetate with cobalt acetoacetonate. J Chem Sci 124:521–527
Semsarzadeh MA, Amiri S (2012) Study of chain sequence in the controlled radical telomerization of vinyl acetate with Co(acac)2 catalyst in bulk. J Polym Res. https://doi.org/10.1007/s10965-012-9891-8
Semsarzadeh MA, Amiri S (2013) Synthesis and characterization of PSt-b-PVAc diblock copolymers via combination of atom transfer radical polymerization and cobalt-mediated radical polymerization. J Polym Res. https://doi.org/10.1007/s10965-013-0139-z
Semsarzadeh MA, Amiri S (2014) Synthesis and characterization of poly(phenylene oxide)- based block copolymers via cobalt mediated radical polymerization (CMRP). Silicon 6:27–34
Semsarzadeh MA, Amiri S (2013) Cobalt mediated radical polymerization of 4-bromo-2,6-dimethyl phenol and its copolymerization with poly(dimethyl siloxane) in the presence of Co(acac)2:DMF catalyst. Silicon Contain Copolym. https://doi.org/10.1007/s12633-014-9185-3
Zhang Q, Li X, Zhao Y, Chen L (2009) Preparation and performance of nanocomposite hydrogels based on different clay. Appl Clay Sci 46:346–350
Zhang J, Wang A (2007) Study on superabsorbent composites. IX: synthesis, characterization and swelling behaviors of polyacrylamide/clay composites based on various clays. React Funct Polym 67:737–745
Aalaie J, Vasheghani-Farahani E, Rahmatpour A, Semsarzadeh A (2008) Effect of montmorillonite on gelation and swelling behavior of sulfonated polyacrylamide nanocomposite hydrogels in electrolyte solutions. Eur Polym J 44:2024–2031
Grinyuk EV, Duk OG, Sheresh IV, Krul LP (2014) Preparation of copolymers of acrylamide and 2-acrylamido-2-methylpropanesulfonic acid by frontal polymerization. Russ J Appl Chem 87:1913–1917
Lin SB, Yuan CH, Ke AR, Quan ZL (2008) Electrical response characterization of PVA–P(AA/AMPS) IPN hydrogels in aqueous Na2SO4 solution. Actuators B-Chem 134:281–286
Durmaz S, Okay O (2000) Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: synthesis and characterization. Polymer 41:3693–3704
Salimi F, Vafaie Sefti M, Jarrahian K, Rafipoor M, Ghorashi SS (2014) Preparation and investigation of the physical and chemical properties of clay-based polyacrylamide/Cr(III) hydrogels as a water shut-off agent in oil reservoir. Korean J Chem Eng 31:986–993
Prud’homme RK, Uhl JT, Poinsatte JP, Halverson F (1983) Rheological monitoring of the formation of polyacrylamide/CrC3 gels. SPE J 23:804–808
Grattoni CA, Al-Sharji HH, Yang C, Muggeridge AH, Zimmerman RW (2001) Rheology and permeability of crosslinked polyacrylamide gel. J Colloid Interface Sci 240:601–607
Vega I, Morris W, Robles J, Peacock H, Marin A (2010) Water shutoff polymer systems: design and efficiency evaluation based on experimental studies. In: Presented at the symposium improved oil recovery, Tulsa, Oklahoma, USA, SPE 129940
Tongwa P, Nygaard R, Bai B (2013) Evaluation of a nanocomposite hydrogel for water shutoff in enhanced oil recovery applications: design, synthesis and characterization. J Appl Polym Sci 128:787–794
Aalaie J, Youssefi M (2012) Study on the dynamic rheometry and swelling properties of the polyacrylamide/laponite nanocomposite hydrogels in electrolyte media. J Macromol Sci Part B 51:1027–1040
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amiri, S. Synthesis and characterization of acrylamide/(3-acrylamidopropyl) trimethyl ammonium chloride solution and acrylamide/Na-montmorillonite hydrogels via controlled radical polymerization for use as high-temperature and high-salinity oil reservoirs. Polym. Bull. 76, 683–699 (2019). https://doi.org/10.1007/s00289-018-2394-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2394-y