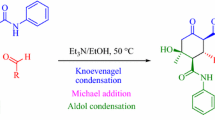
Summary:
2-furfurylmaleimide (FM) was synthesized and characterized unambiguously for the first time by condensing 2-furfurylamine with maleic anhydride to give the corresponding maleamic acid which was in turn cyclized in the presence of acetic anhydride and sodium acetate. A thorough structural assessment was carried out to prove the success of this procedure. FM is highly sensitive to ring-opening hydrolysis by atmospheric moisture and must be kept in a dry medium. This investigation was aimed at using FM as an AB-type monomer in a polycondensation based on the Diels-Alder reaction. Preliminary experiments are reported on this novel system which provided the first polycondensates of this kind.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 January 1998/Accepted: 23 January 1998
Rights and permissions
About this article
Cite this article
Goussé, C., Gandini, A. Synthesis of 2-furfurylmaleimide and preliminary study of its Diels-Alder polycondensation. Polymer Bulletin 40, 389–394 (1998). https://doi.org/10.1007/s002890050267
Issue Date:
DOI: https://doi.org/10.1007/s002890050267