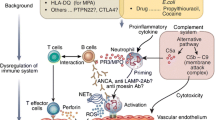
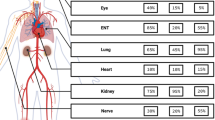
Abstract
Cardiac involvement is common in primary systemic vasculitides and may be due to direct effect of the disease on the heart or due to therapy. We shall review involvement of the heart in the various forms of primary systemic vasculitis. Among anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV), eosinophilic granulomatosis with polyangiitis most commonly involves the heart. Involvement of the heart confers poorer prognosis in AAV, which is also complicated by increased risk of cardiovascular events. Kawasaki’s disease (KD) is the most common form of medium-vessel vasculitis to affect the heart, with coronary artery aneurysms being the most common manifestation. These predispose patients with KD to develop premature ischemic heart disease. Takayasu’s arteritis is the most common large-vessel vasculitis to involve the heart and can result in aortic incompetence, myocarditis, or coronary heart disease. Involvement of the heart in Behcet’s disease is usually in the form of intracardiac mass lesions, thrombosis, or endomyocardial fibrosis. Drugs used in the treatment of systemic vasculitis influence the risk of developing cardiovascular events. Corticosteroid therapy has been shown to increase the risk of myocardial infarction, whereas methotrexate, azathioprine, mycophenolate mofetil, rituximab, and anti-tumor necrosis alpha agents favorably modulate the risk of cardiovascular events, predominantly by dampening systemic inflammation. Awareness of cardiac involvement in vasculitis and accelerated cardiovascular risk in these patients should help clinicians to maximize the modulation of modifiable risk factors for heart disease in these individuals.



Similar content being viewed by others
Abbreviations
- AAV:
-
ANCA-associated small-vessel vasculitis
- ABA:
-
Abatacept
- AMP:
-
Adenosine monophosphate
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- ANCA:
-
Anti-neutrophil cytoplasmic antibodies
- ANCA+:
-
Anti-neutrophil cytoplasmic antibodies positive
- ANCA−:
-
Anti-neutrophil cytoplasmic antibodies negative
- AZA:
-
Azathioprine
- BD:
-
Behcet’s disease
- CANTOS:
-
Canakinumab Anti-inflammatory Thrombosis Outcomes Study
- CMR:
-
Cardiac magnetic resonance
- CRP:
-
C-reactive protein
- CREB:
-
Cyclic AMP-responsive element-binding protein
- CS:
-
Cogan syndrome
- EGPA:
-
Eosinophilic granulomatosis with polyangiitis
- EP:
-
Electrophysiologic
- GCA:
-
Giant cell arteritis
- GPA:
-
Granulomatosis with polyangiitis
- HDL:
-
High-density lipoproteins
- IL:
-
Interleukin
- IL1RA:
-
Interleukin 1 receptor antagonist
- IVIG:
-
Intravenous immunoglobulin
- KD:
-
Kawasaki’s disease
- LDL:
-
Low-density lipoproteins
- MCP1:
-
Monocyte chemoattractant protein 1
- MPA:
-
Microscopic polyangiitis
- MTX:
-
Methotrexate
- MYCOP:
-
Mycophenolate
- NCEP/ATP III:
-
National Cholesterol Education Program/Adult Treatment Panel III
- PAN:
-
Polyarteritis nodosa
- PET:
-
Positron emission tomography
- QTc:
-
Corrected QT interval
- RTX:
-
Rituximab
- STE:
-
Speckle-tracing two-dimensional echocardiography
- TA:
-
Takayasu’s arteritis
- TG:
-
Triglycerides
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TOC:
-
Tocilizumab
References
Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP et al (2010) The rationale for comparative studies of accelerated atherosclerosis in rheumatic diseases. Curr Vasc Pharmacol 8:437–449
Jennette JC, Falk RJ, Bacon PA et al (2013) 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65:1–11. doi:10.1002/art.37715
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. doi:10.1007/s00296-011-1999-3
Hazebroek MR, Kemna MJ, Schalla S et al (2015) Prevalence and prognostic relevance of cardiac involvement in ANCA-associated vasculitis: Eosinophilic granulomatosis with polyangiitis and granulomatosis with polyangiitis. Int J Cardiol 199:170–179. doi:10.1016/j.ijcard.2015.06.087
Guillevin L, Pagnoux C, Seror R et al (2011) The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 90:19–27. doi:10.1097/MD.0b013e318205a4c6
Wallace ZS, Lu N, Unizony S, Stone JH, Choi HK (2015) Improved survival in granulomatosis with polyangiitis: a general population-based study. Semin Arthritis Rheum. doi:10.1016/j.semarthrit.2015.07.009
McGeoch L, Carette S, Cuthbertson D et al (2015) Cardiac involvement in granulomatosis with polyangiitis. J Rheumatol 42:1209–1212. doi:10.3899/jrheum.141513
Pierrot-Deseilligny Despujol C, Pouchot J, Pagnoux C, Coste J, Guillevin L (2010) Predictors at diagnosis of a first Wegener’s granulomatosis relapse after obtaining complete remission. Rheumatology (Oxford) 49:2181–2190. doi:10.1093/rheumatology/keq244
Lacoste C, Mansencal N, M Ben M’rad et al (2011) Valvular involvement in ANCA-associated systemic vasculitis: a case report and literature review. BMC Musculoskelet Disord 12:50. doi:10.1186/1471-2474-12-50
Singh R, Rosen S (2012) Tumor of the heart in a young woman; a rare manifestation of Wegener granulomatosis. Hum Pathol 43:289–292. doi:10.1016/j.humpath.2011.04.020
Misra DP, Chowdhury AC, Edavalath S et al (2016) Endocarditis: the great mimic of rheumatic diseases. Trop Doct. doi:10.1177/0049475515624031
Miszalski-Jamka T, Szczeklik W, Nycz K et al (2012) Two-dimensional speckle-tracking echocardiography reveals systolic abnormalities in granulomatosis with polyangiitis (Wegener’s). Echocardiography 29:803–809. doi:10.1111/j.1540-8175.2012.01699.x
Miszalski-Jamka T, Szczeklik W, Sokolowska B et al (2013) Standard and feature tracking magnetic resonance evidence of myocardial involvement in Churg–Strauss syndrome and granulomatosis with polyangiitis (Wegener’s) in patients with normal electrocardiograms and transthoracic echocardiography. Int J Cardiovasc Imaging 29:843–853. doi:10.1007/s10554-012-0158-6
Miszalski-Jamka T, Szczeklik W, Sokolowska B et al (2011) Cardiac involvement in Wegener’s granulomatosis resistant to induction therapy. Eur Radiol 21:2297–2304. doi:10.1007/s00330-011-2203-6
Guillevin L, Durand-Gasselin B, Cevallos R et al (1999) Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum 42:421–430
Shuai ZW, Lv YF, Zhang MM, Hu ZY (2015) Clinical analysis of patients with myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Genet Mol Res 14:5296–5303. doi:10.4238/2015.May.18.22
Mavrogeni S, Manoussakis MN, Karagiorga TC et al (2009) Detection of coronary artery lesions and myocardial necrosis by magnetic resonance in systemic necrotizing vasculitides. Arthritis Care Res (Hoboken) 61:1121–1129. doi:10.1002/art.24695
Gendelman S, Zeft A, Spalding SJ (2013) Childhood-onset eosinophilic granulomatosis with polyangiitis (formerly Churg–Strauss syndrome): a contemporary single-center cohort. J Rheumatol 40:929–935. doi:10.3899/jrheum.120808
Moosig F, Bremer JP, Hellmich B et al (2013) A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg–Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis 72:1011–1017. doi:10.1136/annrheumdis-2012-201531
Dennert RM, Van Paassen P, Schalla S et al (2010) Cardiac involvement in Churg–Strauss syndrome. Arthritis Rheum 62:627–634. doi:10.1002/art.27263
Vinit J, Bielefeld P, Muller G et al (2010) Heart involvement in Churg–Strauss syndrome: retrospective study in French Burgundy population in past 10 years. Eur J Intern Med 21:341–346. doi:10.1016/j.ejim.2010.05.004
McKinney EF, Willcocks LC, Broecker V, Smith KGC (2014) The immunopathology of ANCA-associated vasculitis. Semin Immunopathol 36:461–478. doi:10.1007/s00281-014-0436-6
Gioffredi A, Maritati F, Oliva E, Buzio C (2014) Eosinophilic granulomatosis with polyangiitis: an overview. Front Immunol 5:549. doi:10.3389/fimmu.2014.00549
Mavrogeni S, Karabela G, Gialafos E et al (2013) Cardiac involvement in ANCA (+) and ANCA (−) Churg–Strauss syndrome evaluated by cardiovascular magnetic resonance. Inflamm Allergy Drug Targets 12:322–327
Marmursztejn J, Guillevin L, Trebossen R et al (2013) Churg–Strauss syndrome cardiac involvement evaluated by cardiac magnetic resonance imaging and positron-emission tomography: a prospective study on 20 patients. Rheumatology (Oxford) 52:642–650. doi:10.1093/rheumatology/kes155
Szczeklik W, Miszalski-Jamka T, Mastalerz L et al (2011) Multimodality assessment of cardiac involvement in Churg–Strauss syndrome patients in clinical remission. Circ J 75:649–655
Marmursztejn J, Cohen P, Duboc D et al (2010) Cardiac magnetic resonance imaging in Churg–Strauss-syndrome. Impact of immunosuppressants on outcome assessed in a prospective study on 8 patients. Clin Exp Rheumatol 28:8–13
Miszalski-Jamka T, Szczeklik W, Nycz K et al (2012) The mechanics of left ventricular dysfunction in patients with Churg–Strauss syndrome. Echocardiography 29:568–578. doi:10.1111/j.1540-8175.2011.01654.x
Szczeklik W, Sokolowska BM, Mastalerz L et al (2011) QT dispersion in patients with Churg–Strauss syndrome. Kardiol Pol 69:1143–1149
Groh M, Masciocco G, Kirchner E et al (2014) Heart transplantation in patients with eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome). J Heart Lung Transplant 33:842–850. doi:10.1016/j.healun.2014.02.023
Morgan MD, Turnbull J, Selamet U et al (2009) Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum 60:3493–3500. doi:10.1002/art.24957
Chironi G, Pagnoux C, Simon A et al (2007) Increased prevalence of subclinical atherosclerosis in patients with small-vessel vasculitis. Heart 93:96–99. doi:10.1136/hrt.2006.088443
Terrier B, Chironi G, Pagnoux C et al (2014) Factors associated with major cardiovascular events in patients with systemic necrotizing vasculitides: results of a longterm followup study. J Rheumatol 41:723–729. doi:10.3899/jrheum.130882
Bae YD, Choi HJ, Lee JC et al (2006) Clinical features of polyarteritis nodosa in Korea. J Korean Med Sci 21:591–595
Schrader ML, Hochman JS, Bulkley BH (1985) The heart in polyarteritis nodosa: a clinicopathologic study. Am Heart J 109:1353–1359
Gunal N, Kara N, Cakar N et al (1997) Cardiac involvement in childhood polyarteritis nodosa. Int J Cardiol 60:257–262
Zoller B, Li X, Sundquist J, Sundquist K (2012) Risk of subsequent coronary heart disease in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. PLoS One 7:e33442. doi:10.1371/journal.pone.0033442
Bourgarit A, Le Toumelin P, Pagnoux C et al (2005) Deaths occurring during the first year after treatment onset for polyarteritis nodosa, microscopic polyangiitis, and Churg–Strauss syndrome: a retrospective analysis of causes and factors predictive of mortality based on 595 patients. Medicine (Baltimore) 84:323–330
Fortin PR, Larson MG, Watters AK et al (1995) Prognostic factors in systemic necrotizing vasculitis of the polyarteritis nodosa group—a review of 45 cases. J Rheumatol 22:78–84
Guillevin L, Lhote F, Gayraud M et al (1996) Prognostic factors in polyarteritis nodosa and Churg–Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 75:17–28
Singh S, Vignesh P, Burgner D (2015) The epidemiology of Kawasaki disease: a global update. Arch Dis Child 100:1084–1088. doi:10.1136/archdischild-2014-307536
Han BK, Lesser A, Rosenthal K et al (2014) Coronary computed tomographic angiographic findings in patients with Kawasaki disease. Am J Cardiol 114:1676–1681. doi:10.1016/j.amjcard.2014.09.004
Tacke CE, Kuipers IM, Groenink M, Spijkerboer AM, Kuijpers TW (2011) Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging 4:712–720. doi:10.1161/circimaging.111.965996
Chaudhuri K, Singh Ahluwalia T, Singh S, Binepal G, Khullar M (2011) Polymorphism in the promoter of the CCL5 gene (CCL5G-403A) in a cohort of North Indian children with Kawasaki disease. A preliminary study. Clin Exp Rheumatol 29:S126–S130
Caballero-Mora FJ, Alonso-Martin B, Tamariz-Martel-Moreno A, Cano-Fernandez J, Sanchez-Bayle M (2011) Kawasaki disease in 76 patients. Risk factors for coronary artery aneurysms. An Pediatr (Barc) 74:232–238. doi:10.1016/j.anpedi.2010.11.024
Callinan LS, Tabnak F, Holman RC et al (2012) Kawasaki syndrome and factors associated with coronary artery abnormalities in California. Pediatr Infect Dis J 31:894–898. doi:10.1097/INF.0b013e31825c4d7c
Kim JJ, Hong YM, Yun SW et al (2012) Assessment of risk factors for Korean children with Kawasaki disease. Pediatr Cardiol 33:513–520. doi:10.1007/s00246-011-0143-1
Falcini F, Rigante D, Masi L et al (2013) Fibroblast growth factor 23 (FGF23) gene polymorphism in children with Kawasaki syndrome (KS) and susceptibility to cardiac abnormalities. Ital J Pediatr 39:69. doi:10.1186/1824-7288-39-69
Giannouli G, Tzoumaka-Bakoula C, Kopsidas I et al (2013) Epidemiology and risk factors for coronary artery abnormalities in children with complete and incomplete Kawasaki disease during a 10-year period. Pediatr Cardiol 34:1476–1481. doi:10.1007/s00246-013-0673-9
Ruan Y, Ye B, Zhao X (2013) Clinical characteristics of Kawasaki syndrome and the risk factors for coronary artery lesions in China. Pediatr Infect Dis J 32:e397–e402. doi:10.1097/INF.0b013e31829dd45e
Maric LS, Knezovic I, Papic N et al (2015) Risk factors for coronary artery abnormalities in children with Kawasaki disease: a 10-year experience. Rheumatol Int 35:1053–1058. doi:10.1007/s00296-014-3186-9
Kuwabara M, Yashiro M, Kotani K et al (2015) Cardiac lesions and initial laboratory data in Kawasaki disease: a nationwide survey in Japan. J Epidemiol 25:189–193. doi:10.2188/jea.JE20140128
Ye Q, Shao WX, Shang SQ et al (2015) A comprehensive assessment of the value of laboratory indices in diagnosing Kawasaki disease. Arthritis Rheumatol 67:1943–1950. doi:10.1002/art.39112
Lin YJ, Chang JS, Liu X et al (2015) Genetic variants in PLCB4/PLCB1 as susceptibility loci for coronary artery aneurysm formation in Kawasaki disease in Han Chinese in Taiwan. Sci Rep 5:14762. doi:10.1038/srep14762
Zhao LL, Wang YB, Suo L (2011) Meta-analysis of the risk factors for coronary artery lesion secondary to Kawasaki disease in Chinese children. Zhonghua Er Ke Za Zhi 49:459–467
Orenstein JM, Shulman ST, Fox LM et al (2012) Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS One 7:e38998. doi:10.1371/journal.pone.0038998
Lin IC, Kuo HC, Lin YJ et al (2012) Augmented TLR2 expression on monocytes in both human Kawasaki disease and a mouse model of coronary arteritis. PLoS One 7:e38635. doi:10.1371/journal.pone.0038635
Lin IC, Suen JL, Huang SK et al (2013) Dectin-1/Syk signaling is involved in Lactobacillus casei cell wall extract-induced mouse model of Kawasaki disease. Immunobiology 218:201–212. doi:10.1016/j.imbio.2012.04.004
Nishio H, Kanno S, Onoyama S et al (2011) Nod1 ligands induce site-specific vascular inflammation. Arterioscler Thromb Vasc Biol 31:1093–1099. doi:10.1161/ATVBAHA.110.216325
Nakamura Y, Aso E, Yashiro M et al (2013) Mortality among Japanese with a history of Kawasaki disease: results at the end of 2009. J Epidemiol 23:429–434
Niedra E, Chahal N, Manlhiot C, Yeung RS, McCrindle BW (2014) Atorvastatin safety in Kawasaki disease patients with coronary artery aneurysms. Pediatr Cardiol 35:89–92. doi:10.1007/s00246-013-0746-9
Mori M, Imagawa T, Hara R et al (2012) Efficacy and limitation of infliximab treatment for children with Kawasaki disease intractable to intravenous immunoglobulin therapy: report of an open-label case series. J Rheumatol 39:864–867. doi:10.3899/jrheum.110877
Tremoulet AH, Jain S, Jaggi P et al (2014) Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet 383:1731–1738. doi:10.1016/s0140-6736(13)62298-9
Millar K, Manlhiot C, Yeung RS, Somji Z, McCrindle BW (2012) Corticosteroid administration for patients with coronary artery aneurysms after Kawasaki disease may be associated with impaired regression. Int J Cardiol 154:9–13. doi:10.1016/j.ijcard.2010.08.070
Adachi S, Sakaguchi H, Kuwahara T et al (2010) High regression rate of coronary aneurysms developed in patients with immune Globulin-Resistant Kawasaki disease treated with steroid pulse therapy. Tohoku J Exp Med 220:285–290. doi:10.1620/tjem.220.285
Chen S, Dong Y, Yin Y, Krucoff MW (2013) Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart 99:76–82. doi:10.1136/heartjnl-2012-302126
Harada M, Yokouchi Y, Oharaseki T et al (2012) Histopathological characteristics of myocarditis in acute-phase Kawasaki disease. Histopathology 61:1156–1167. doi:10.1111/j.1365-2559.2012.04332.x
Tacke CE, Romeih S, Kuipers IM et al (2013) Evaluation of cardiac function by magnetic resonance imaging during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging 6:67–73. doi:10.1161/circimaging.112.976969
McCandless RT, Minich LL, Wilkinson SE et al (2013) Myocardial strain and strain rate in Kawasaki disease. Eur Heart J Cardiovasc Imaging 14:1061–1068. doi:10.1093/ehjci/jet041
Ichida F, Fatica NS, O’Loughlin JE et al (1988) Correlation of electrocardiographic and echocardiographic changes in Kawasaki syndrome. Am Heart J 116:812–819
Ghelani SJ, Singh S, Manojkumar R (2011) QT interval dispersion in North Indian children with Kawasaki disease without overt coronary artery abnormalities. Rheumatol Int 31:301–305. doi:10.1007/s00296-009-1252-5
Oyamada J, Toyono M, Shimada S, Aoki-Okazaki M, Takahashi T (2012) Altered central aortic elastic properties in Kawasaki disease are related to changes in left ventricular geometry and coronary artery aneurysm formation. J Am Soc Echocardiogr 25:690–696. doi:10.1016/j.echo.2012.03.003
AlHuzaimi A, Al Mashham Y, Potts JE, De Souza AM, Sandor GG (2013) Echo-Doppler assessment of arterial stiffness in pediatric patients with Kawasaki disease. J Am Soc Echocardiogr 26:1084–1089. doi:10.1016/j.echo.2013.05.015
Shah V, Christov G, Mukasa T et al (2015) Cardiovascular status after Kawasaki disease in the UK. Heart 101:1646–1655. doi:10.1136/heartjnl-2015-307734
Ishikawa K, Maetani S (1994) Long-term outcome for 120 Japanese patients with Takayasu’s disease. Clinical and statistical analyses of related prognostic factors. Circulation 90:1855–1860
Endo M, Tomizawa Y, Nishida H et al (2003) Angiographic findings and surgical treatments of coronary artery involvement in Takayasu arteritis. J Thorac Cardiovasc Surg 125:570–577. doi:10.1067/mtc.2003.39
Comarmond C, Cluzel P, Toledano D et al (2014) Findings of cardiac magnetic resonance imaging in asymptomatic myocardial ischemic disease in Takayasu arteritis. Am J Cardiol 113:881–887. doi:10.1016/j.amjcard.2013.11.045
Lee GY, Jang SY, Ko SM et al (2012) Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: analysis of 204 Korean patients at a single center. Int J Cardiol 159:14–20. doi:10.1016/j.ijcard.2011.01.094
Li C, Liu Y, Qi R et al (2013) Repair of aortic regurgitation due to Takayasu arteritis. Heart Surg Forum 16:E24–E26. doi:10.1532/hsf98.20121059
Misra R, Aggarwal A, Chag M, Sinha N, Shrivastava S (1994) Raised anticardiolipin antibodies in Takayasu’s arteritis. Lancet 343:1644–1645
Jordan NP, Bezanahary H, D’Cruz DP (2015) Increased risk of vascular complications in Takayasu’s arteritis patients with positive lupus anticoagulant. Scand J Rheumatol 44:211–214. doi:10.3109/03009742.2014.964305
Castellani M, Vanoli M, Cali G et al (2001) Ventilation-perfusion lung scan for the detection of pulmonary involvement in Takayasu’s arteritis. Eur J Nucl Med 28:1801–1805. doi:10.1007/s002590100648
Wang X, Dang A, Chen B, Lv N, Liu Q (2015) Takayasu arteritis-associated pulmonary hypertension. J Rheumatol 42:495–503. doi:10.3899/jrheum.140436
Dong H, Jiang X, Peng M et al (2014) Percutaneous transluminal angioplasty for symptomatic pulmonary stenosis in Takayasu arteritis. J Rheumatol 41:1856–1862. doi:10.3899/jrheum.131007
Kim GB, Kwon BS, Bae EJ, Noh CI (2012) Takayasu arteritis presenting as dilated cardiomyopathy with left ventricular thrombus in association with ulcerative colitis. J Am Coll Cardiol 60:e25. doi:10.1016/j.jacc.2011.11.080
Seker T, Baykan AO, Borekci A, Gur M, Cayli M (2014) Successful treatment of a huge thrombus with thrombolytic therapy in a patient with normal left ventricle function and Takayasu arteritis. Turk Kardiyol Dern Ars 42:763–766. doi:10.5543/tkda.2014.60687
da Silva TF, Levy-Neto M, Bonfa E, Pereira RM (2013) High prevalence of metabolic syndrome in Takayasu arteritis: increased cardiovascular risk and lower adiponectin serum levels. J Rheumatol 40:1897–1904. doi:10.3899/jrheum.130162
Matsue Y, Ohno M, Nagahori W et al (2011) A case of giant cell arteritis with massive pericardial effusion. Heart Vessels 26:562–564. doi:10.1007/s00380-010-0101-5
Kimura T, Komura M, Okubo Y (2012) Atypical giant cell arteritis predominantly involving intramural coronary arteries: a case showing refractory dialysis-related hypotension. Heart Vessels 27:216–220. doi:10.1007/s00380-011-0158-9
Strecker T, Zimmermann S, Wachter DL, Agaimy A (2011) Aortic dissection caused by giant cell arteritis. Heart Surg Forum 14:E137–E138. doi:10.1532/hsf98.20101114
Tomasson G, Peloquin C, Mohammad A et al (2014) Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med 160:73–80. doi:10.7326/m12-3046
Schmidt J, Kermani TA, Muratore F et al (2013) Statin use in giant cell arteritis: a retrospective study. J Rheumatol 40:910–915. doi:10.3899/jrheum.121150
Gasparovic H, Djuric Z, Bosnic D et al (2011) Aortic root vasculitis associated with Cogan’s syndrome. Ann Thorac Surg 92:340–341. doi:10.1016/j.athoracsur.2010.12.068
Weyn T, Haine S, Conraads V (2009) Cogan’s syndrome with left main coronary artery occlusion. Cardiol J 16:573–576
Geri G, Wechsler B, Thi Huong du L et al (2012) Spectrum of cardiac lesions in Behcet disease: a series of 52 patients and review of the literature. Medicine (Baltimore) 91:25–34. doi:10.1097/MD.0b013e3182428f49
Bonitsis NG, Luong Nguyen LB, LaValley MP et al (2015) Gender-specific differences in Adamantiades–Behcet’s disease manifestations: an analysis of the German registry and meta-analysis of data from the literature. Rheumatology (Oxford) 54:121–133. doi:10.1093/rheumatology/keu247
Emmungil H, Yasar Bilge NS, Kucuksahin O et al (2014) A rare but serious manifestation of Behcet’s disease: intracardiac thrombus in 22 patients. Clin Exp Rheumatol 32:S87–S92
La Regina M, Gasparyan AY, Orlandini F, Prisco D (2010) Behcet’s disease as a model of venous thrombosis. Open Cardiovasc Med J 4:71–77. doi:10.2174/1874192401004020071
Yue C, Li J, Li M et al (2012) Cardiac mass in Behcet’s disease. Clin Exp Rheumatol 30:S27–S31
Ma WG, Zheng J, Zhu JM et al (2012) Aortic regurgitation caused by Behcet’s disease: surgical experience during an 11-year period. J Card Surg 27:39–44. doi:10.1111/j.1540-8191.2011.01392.x
Ulusan Z, Karadag AS, Tasar M, Kalender M, Darcin OT (2014) Behcet’s disease and cardiovascular involvement: our experience of asymptomatic Behcet’s patients. Cardiovasc J Afr 25:63–66. doi:10.5830/CVJA-2014-003
Demirelli S, Degirmenci H, Bilen H et al (2014) Left ventricular mechanics in Behcet’s disease: a speckle tracking echocardiographic study. Bosn J Basic Med Sci 14:160–164. doi:10.17305/bjbms.2014.3.2
Yagmur J, Sener S, Acikgoz N et al (2011) Subclinical left ventricular dysfunction in Behcet’s disease assessed by two-dimensional speckle tracking echocardiography. Eur J Echocardiogr 12:536–541. doi:10.1093/ejechocard/jer088
Cobankara V, Guclu A, Kuru O et al (2012) Evaluation of biventricular myocardial performance index in patients with Behcet’s disease. J Int Med Res 40:328–332
Cansel M, Yagmur J, Tasolar H et al (2014) Assessment of atrial conduction time in patients with Behcet’s disease. Acta Reumatol Port 39:29–36
Sayin MR, Akpinar I, Gursoy YC et al (2013) Assessment of QRS duration and presence of fragmented QRS in patients with Behcet’s disease. Coron Artery Dis 24:398–403. doi:10.1097/MCA.0b013e328361a978
Karabag T, Aydin M, Dogan SM et al (2012) Investigation of the atrial electromechanical delay duration in Behcet patients by tissue Doppler echocardiography. Eur Heart J Cardiovasc Imaging 13:251–256. doi:10.1093/ejechocard/jer227
Borman P, Tuncay F, Kocaoglu S et al (2012) The subclinic autonomic dysfunction in patients with Behcet disease: an electrophysiological study. Clin Rheumatol 31:41–47. doi:10.1007/s10067-011-1763-9
Pandey A, Garg J, Krishnamoorthy P et al (2014) Predictors of coronary artery disease in patients with Behçet’s disease. Cardiology (Switzerland) 129:203–206. doi:10.1159/000365139
Tasolar H, Tasolar S, Kurtulus D et al (2014) Increased epicardial adipose tissue thickness on transthoracic echocardiography in patients with Behcet disease. J Ultrasound Med 33:1393–1400. doi:10.7863/ultra.33.8.1393
Kocabay G, Hasdemir H, Yildiz M (2012) Evaluation of pulse wave velocity in systemic lupus erythematosus, rheumatoid arthritis and Behcet’s disease. J Cardiol 59:72–77. doi:10.1016/j.jjcc.2011.09.004
Balta I, Balta S, Koryurek OM et al (2014) Mean platelet volume is associated with aortic arterial stiffness in patients with Behcet’s disease without significant cardiovascular involvement. J Eur Acad Dermatol Venereol 28:1388–1393. doi:10.1111/jdv.12297
Botsios C, Sfriso P, Punzi L, Todesco S (2007) Non-complementaemic urticarial vasculitis: successful treatment with the IL-1 receptor antagonist, anakinra. Scand J Rheumatol 36:236–237. doi:10.1080/03009740600938647
Boyer EM, Turman M, O’Neil KM (2011) Partial response to anakinra in life-threatening Henoch–Schönlein purpura: case report. Pediatr Rheumatol Online J 9:21. doi:10.1186/1546-0096-9-21
Wei L, MacDonald TM, Walker BR (2004) Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 141:764–770
Souverein PC, Berard A, Van Staa TP et al (2004) Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 90:859–865. doi:10.1136/hrt.2003.020180
Varas-Lorenzo C, Rodriguez LA, Maguire A, Castellsague J, Perez-Gutthann S (2007) Use of oral corticosteroids and the risk of acute myocardial infarction. Atherosclerosis 192:376–383. doi:10.1016/j.atherosclerosis.2006.05.019
Dadfarmay S, Berkowitz R, Kim B (2012) Irreversible end-stage cardiomyopathy following a single dose of cyclophosphamide. Congest Heart Fail 18:234–237. doi:10.1111/j.1751-7133.2011.00279.x
Agarwal N, Burkart TA (2013) Transient, high-grade atrioventricular block from high-dose cyclophosphamide. Tex Heart Inst J 40:626–627
Zhou F, Ling C, Guo L et al (2014) Continuous low-dose cyclophosphamide and prednisone in the treatment of relapsed/refractory multiple myeloma with severe heart failure. Leuk Lymphoma 55:2271–2276. doi:10.3109/10428194.2014.887711
Ajeganova S, de Faire U, Jogestrand T, Frostegard J, Hafstrom I (2012) Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis—an inception cohort study. J Rheumatol 39:1146–1154. doi:10.3899/jrheum.111334
Ahlehoff O, Skov L, Gislason G et al (2015) Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol 29:1128–1134. doi:10.1111/jdv.12768
Westlake SL, Colebatch AN, Baird J et al (2010) The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 49:295–307. doi:10.1093/rheumatology/kep366
Micha R, Imamura F, Wyler von Ballmoos M et al (2011) Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 108:1362–1370. doi:10.1016/j.amjcard.2011.06.054
De Vecchis R, Baldi C, Palmisani L (2015) Protective effects of methotrexate against ischemic cardiovascular disorders in patients treated for rheumatoid arthritis or psoriasis: novel therapeutic insights coming from a meta-analysis of the literature data. Anatol J Cardiol. doi:10.5152/akd.2015.6136
Thornton CC, Al-Rashed F, Calay D et al (2015) Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: a novel mechanism for vascular protection in chronic systemic inflammation. Ann Rheum Dis. doi:10.1136/annrheumdis-2014-206305
Ronda N, Greco D, Adorni MP et al (2015) Newly identified antiatherosclerotic activity of methotrexate and adalimumab: complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol 67:1155–1164. doi:10.1002/art.39039
Bulgarelli A, Leite AC Jr, Dias AA, Maranhao RC (2013) Anti-atherogenic effects of methotrexate carried by a lipid nanoemulsion that binds to LDL receptors in cholesterol-fed rabbits. Cardiovasc Drugs Ther 27:531–539. doi:10.1007/s10557-013-6488-3
Moreira DM, Lueneberg ME, da Silva RL, Fattah T, Mascia Gottschall CA (2013) Rationale and design of the TETHYS trial: the effects of methotrexate therapy on myocardial infarction with ST-segment elevation. Cardiology 126:167–170. doi:10.1159/000351972
Zanoli L, Rastelli S, Inserra G et al (2014) Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs. Atherosclerosis 234:346–351. doi:10.1016/j.atherosclerosis.2014.03.023
Pols TW, Bonta PI, Pires NM et al (2010) 6-Mercaptopurine inhibits atherosclerosis in apolipoprotein e*3-leiden transgenic mice through atheroprotective actions on monocytes and macrophages. Arterioscler Thromb Vasc Biol 30:1591–1597. doi:10.1161/atvbaha.110.205674
Spagnoletti G, Citterio F, Favi E et al (2009) Cardiovascular risk profile in kidney transplant recipients treated with two immunosuppressive regimens: tacrolimus and mycophenolate mofetil versus everolimus and low-dose cyclosporine. Transpl Proc 41:1175–1177. doi:10.1016/j.transproceed.2009.03.045
Cuervas-Mons V, Herrero JI, Gomez MA et al (2015) Impact of tacrolimus and mycophenolate mofetil regimen vs. a conventional therapy with steroids on cardiovascular risk in liver transplant patients. Clin Transplant 29:667–677. doi:10.1111/ctr.12557
van Leuven SI, Mendez-Fernandez YV, Wilhelm AJ et al (2012) Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus-prone LDLr(−/−) mice. Ann Rheum Dis 71:408–414. doi:10.1136/annrheumdis-2011-200071
van Sijl AM, van der Weele W, Nurmohamed MT (2014) Myocardial infarction after rituximab treatment for rheumatoid arthritis: is there a link? Curr Pharm Des 20:496–499
Passalia C, Minetto P, Arboscello E et al (2013) Cardiovascular adverse events complicating the administration of rituximab: report of two cases. Tumori 99:288e–292e. doi:10.1700/1390.15471
Fernandez-Nebro A, Marenco JL, Lopez-Longo F et al (2014) The effects of rituximab on the lipid profile of patients with active systemic lupus erythematosus: results from a nationwide cohort in Spain (LESIMAB). Lupus 23:1014–1022. doi:10.1177/0961203314534909
Raterman HG, Levels H, Voskuyl AE et al (2013) HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis 72:560–565. doi:10.1136/annrheumdis-2011-201228
Provan SA, Berg IJ, Hammer HB et al (2015) The impact of newer biological disease modifying anti-rheumatic drugs on cardiovascular risk factors: a 12-month longitudinal study in rheumatoid arthritis patients treated with rituximab, abatacept and tociliziumab. PLoS One 10:e0130709. doi:10.1371/journal.pone.0130709
Hsue PY, Scherzer R, Grunfeld C et al (2014) Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc 3:e001267. doi:10.1161/jaha.114.001267
Clifford A, Hoffman GS (2014) Recent advances in the medical management of Takayasu arteritis: an update on use of biologic therapies. Curr Opin Rheumatol 26:7–15. doi:10.1097/bor.0000000000000004
Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323:236–241. doi:10.1056/nejm199007263230405
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107:3133–3140. doi:10.1161/01.cir.0000077913.60364.d2
Solomon DH, Rassen JA, Kuriya B et al (2013) Heart failure risk among patients with rheumatoid arthritis starting a TNF antagonist. Ann Rheum Dis 72:1813–1818. doi:10.1136/annrheumdis-2012-202136
Westlake SL, Colebatch AN, Baird J et al (2011) Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 50:518–531. doi:10.1093/rheumatology/keq316
Roubille C, Richer V, Starnino T et al (2015) The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 74:480–489. doi:10.1136/annrheumdis-2014-206624
Souto A, Salgado E, Maneiro JR et al (2015) Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol 67:117–127. doi:10.1002/art.38894
Strang AC, Bisoendial RJ, Kootte RS et al (2013) Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 229:174–181. doi:10.1016/j.atherosclerosis.2013.04.031
Schultz O, Oberhauser F, Saech J et al (2010) Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One 5:e14328. doi:10.1371/journal.pone.0014328
Kume K, Amano K, Yamada S et al (2011) Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomized controlled trial. J Rheumatol 38:2169–2171. doi:10.3899/jrheum.110340
Lazzerini PE, Acampa M, Capecchi PL et al (2015) Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res (Hoboken) 67:332–339. doi:10.1002/acr.22455
Ursini F, Mauro D, Naty S, Gagliardi D, Grembiale RD (2012) Improvement in insulin resistance after short-term treatment with abatacept: case report and short review. Clin Rheumatol 31:1401–1402. doi:10.1007/s10067-012-2034-0
Ursini F, Russo E, Letizia Hribal M et al (2015) Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore) 94:e888. doi:10.1097/md.0000000000000888
Mathieu S, Couderc M, Glace B et al (2013) Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics 7:259–264. doi:10.2147/btt.s52003
Vanrenterghem Y, Bresnahan B, Campistol J et al (2011) Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies). Transplantation 91:976–983. doi:10.1097/TP.0b013e31820c10eb
Nowak C, Sundstrom J, Gustafsson S et al (2016) Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes 65:276–284. doi:10.2337/db15-0881
Fragoso JM, Delgadillo H, Llorente L et al (2010) Interleukin 1 receptor antagonist polymorphisms are associated with the risk of developing acute coronary syndrome in Mexicans. Immunol Lett 133:106–111. doi:10.1016/j.imlet.2010.08.003
Johansen NB, Vistisen D, Brunner EJ et al (2012) Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PLoS One 7:e37165. doi:10.1371/journal.pone.0037165
Ikonomidis I, Tzortzis S, Lekakis J et al (2011) Association of soluble apoptotic markers with impaired left ventricular deformation in patients with rheumatoid arthritis. Effects of inhibition of interleukin-1 activity by anakinra. Thromb Haemost 106:959–967. doi:10.1160/th11-02-0117
Ridker PM, Howard CP, Walter V et al (2012) Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 126:2739–2748. doi:10.1161/circulationaha.112.122556
Ridker PM (2013) Closing the loop on inflammation and atherothrombosis: why perform the CIRT and CANTOS trials? Trans Am Clin Climatol Assoc 124:174–190
Acknowledgments
The authors would like to acknowledge Dr Vikas Agarwal and Professor Vir Singh Negi for their encouragement and suggestions toward improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Misra, D.P., Shenoy, S.N. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol Int 37, 151–167 (2017). https://doi.org/10.1007/s00296-016-3435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3435-1