Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the coronavirus disease 19 (COVID-19) pandemic, which is deeply affecting the whole world. In this new case for the scientific world, scientists are investigating the etiopathogenesis of viral infection-induced damage and have started to focus on the short and long-term immune system effects and alterations after SARS-CoV-2 infection. The case is here reported of a 53-year-old female patient with acute monoarthritis after SARS-CoV-2 infection, who responded adequately to 150 mg/day diclofenac treatment, and the available case reports are comprehensively reviewed. With the focus on arthritis after SARS-CoV2 infection, which emerges as a new pathological condition associated with COVID-19, it was aimed to examine the possible immunological mechanisms of post-COVID-19 arthritis based on the current data on SARS-CoV-2 and the known pathogenetic background of viral arthritis.
Similar content being viewed by others
Introduction
Since its first appearance in Wuhan, China, SARS-CoV-2, a new type of coronavirus, has surrounded and shaken the world with the influence of various mutations and new variants [1]. COVID-19 has a heterogeneous clinical course; the asymptomatic course is on one side of the clinical spectrum, and multiple organ failure on the other. In susceptible individuals, an overabundant immune reaction, as well as the lytic impact of the virus on cells, may contribute to the emergence of severe clinical symptoms [2, 3]. COVID-19 can lead to a wide variety of complications affecting pulmonary, neurological, cardiovascular, rheumatological, dermatological, and many other systems. It is not yet clear how these complications will progress and whether they are reversible or not [4].
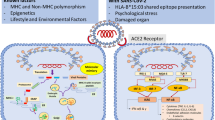
It has been argued that there is a potential relationship between different viral agents, encompassing Epstein-Barr virus, cytomegalovirus, hepatitis C, parvovirus B19, influenza, and activated autoimmunity [5]. SARS-CoV-2 stimulates interleukin-6-related pathways and induces cytokine storm and macrophage activation syndrome by this mechanism [6]. Additionally, it can influence the antigen presentation as well as differentiate interferon-dependent pathways [7].
The relationship between various infections and joint involvement has been well defined, particularly based on reactive arthritis (ReA). The spondyloarthritis spectrum includes ReA, which is defined as arthritis that mostly occurs after genitourinary or enteric infection [8]. It has the clinical nature of arthritis induced by infection of a tissue distant to the joint rather than an infection affecting primary joint structures [9]. Although the etiopathogenesis of ReA has not been fully revealed, it is thought to be the result of a complex process that passes between triggering environmental factors (infectious agents) and genetic characteristics [10]. Although various rheumatic and musculoskeletal disorders associated with COVID-19 have been reported [11, 12], there have been no controlled clinical studies on ReA associated with COVID-19. In this paper, the case is presented of a patient who developed ReA after COVID-19. It was also performed a comprehensive review of the literature and compile COVID-19-related ReA cases, to be able to describe the general characteristics of this patient group.
Case presentation
A 53-year-old female patient with a history of hypertension was admitted to the Emergency Department (ED) in February 2021 with complaints of headache and loss of taste. The SARS-CoV-2 PCR test on nasopharyngeal swab sample performed in the emergency room was positive. The patient, without hyperpyrexia, dyspnea, cough, asthenia, and lung signs at that time, was not hospitalized. After 12 days, the patient presented at the ED again due to worsening of the clinical condition, cough, sputum, and dyspnea. Bilateral ground-glass opacity was observed on lung imaging. Blood tests supported inflammatory status with C-reactive protein (CRP) 36.15 mg/l (< 10 mg/l), sedimentation 96 mm/h (< 16 mm/h), hemoglobin (Hb) 11.57 g/dl (11–16 g/dl), white blood cells (WBC) 11,150/µl (4000–10,000/µl), neutrophil 8220/µl (1500–5700/µl), lymphocyte 2090/µl (600–4000/µl), ferritin 318.6 ng/ml (13–150 ng/ml), and D-dimer 3112 ng/ml (0–630 ng/ml). The patient was hospitalized in the pulmonary medicine department. The nasopharyngeal swab test was repeated and positive. The patient was treated with favipiravir, hydroxychloroquine, azithromycin, anticoagulant (for thromboembolic event prophylaxis), and oxygen support. On the 15th day of hospitalization, the patient's complaints decreased significantly, she did not need oxygen support, and her general condition improved, so she was discharged. The results of the laboratory were as follows: Hb 12.81 (11–16 g/dl), WBC 8900/µl (4000–10,000/µl), neutrophil 5963/µl (1500–5700/µl), lymphocyte 1958/µl (600–4000/µl), platelet 364,000 (100,000/400000/µl), CRP 0.78 mg/l (< 10 mg/l), sedimentation 18 (< 16 mm/h), uric acid 5.9 mg/dl (3,4–7 mg/dl), creatinine 0.51 mg/dl (0.7–1.2 mg/dl), ALT 22.20 U/l (0–41 U/l).
On the 14th day after discharge, the patient presented at the Physical Medicine and Rehabilitation Clinic with complaints of pain and swelling in the left knee, morning stiffness, and limitation of joint movement. The patient had no history of rheumatic disease, family history of rheumatic disease, or trauma. She did not report any other recent history of infection or symptoms. There was no history of ocular signs suggestive of conjunctivitis or uveitis, and no history of inflammatory diarrhea or psoriasis. The patient's gait was antalgic and physical examination revealed tenderness, swelling, and redness in the left knee. Left knee flexion and extension measured using a goniometer were recorded as 115° and 140°, respectively. Knee radiography was assessed and no evidence was found to support the patient's clinical findings. Ultrasonography revealed effusion in the left knee, which was evaluated as arthritis (Fig. 1). Arthrocentesis was performed and 12 ml cloudy-yellow synovial fluid was aspirated (18.000/mm3 WBC of which 80% polymorphonucleates). The synovial fluid culture was negative for microbiological agents, and no crystals were detected. Extra-articular manifestations were not detected. In laboratory tests, the results were as follows: Hb 13.62 (11–16 g/dl), WBC 10,610/µl (4000–10,000/µl), neutrophil 5919/µl (1500–5700/µl), lymphocyte 3714/µl (600–4000/µl), platelet 362,000 (100,000/400000/µl), CRP 10.8 mg/l (< 10 mg/l), sedimentation 30 (< 16 mm/h), uric acid 5.8 mg/dl (3,4–7 mg/dl), creatinine 0.57 mg/dl (0.7–1.2 mg/dl), ALT 26.22 U/l (0–41 U/l), rheumatoid factor (RF) negative, anti-citrullinated protein/peptide antibody (ACPA) negative, antinuclear antibody (ANA) negative, and human leukocyte antigen B27 (HLA-B27) negative. Urine and blood cultures were evaluated and no signs of infection were detected. No increase in procalcitonin value was determined. A urethral swab sample was taken and a gram-stained smear was examined under a microscope. The urethral swab cultures and antigen tests were evaluated, stool cultures were assessed, and no infection was determined in the results.
There are no established diagnostic or classification criteria for ReA, but the American College of Rheumatology (ACR) proposed general principles in a 1999 workshop [13]. Considering all these evaluations, it was decided that the most likely diagnosis was ReA after SARS-CoV-2 infection. Diclofenac 150 mg/day treatment was started. On the 10th day of treatment, the patient's clinical and laboratory findings partially improved. Medical treatment was continued for six weeks, then tapered, and finally discontinued. Arthritis and inflammatory signs were not observed in the follow-up examinations (Fig. 2). Significant regression in edema was confirmed by ultrasonographic evaluation in this period. Knee range of motion was increased (knee flexion 130° and knee extension 145°). The antalgic gait pattern improved, and there was no tenderness or redness in the left knee. The follow-up laboratory results were as follows: Hb 13.14 (11–16 g/dl), WBC 9200/µl (4000–10,000/µl), neutrophil 5640/µl (1500–5700/µl), lymphocyte 3512/µl (600–4000/µl), platelet 344,000 (100,000/400000/µl), CRP 2.4 mg/l (< 10 mg/l), sedimentation 14 (< 16 mm/h), uric acid 6.1 mg/dl (3,4–7 mg/dl), creatinine 0.68 mg/dl (0.7–1.2 mg/dl), ALT 36.40 U/l (0–41 U/l).
Search strategy and case selection
A case-based search was conducted according to the review strategies recommended in the literature [14]. Web of Science, Scopus, MEDLINE/Pubmed, DOAJ, and OUCI databases were comprehensively searched. The available literature was searched with the following keywords: ‘COVID-19’ OR ‘SARS-CoV-2’ AND ‘reactive arthritis’ OR ‘acute arthritis’. Case reports in English were reviewed in patients aged ≥ 18 years. Cases were excluded if the diagnosis of COVID-19 was not made from a nasopharyngeal swab test or serological examination. COVID-19 evaluation with only clinical and radiological findings was not considered sufficient. Papers that did not present a case and were published as a letter in reply to a previous article were excluded. Cases that were thought to be drug-induced arthritis or when the ReA diagnosis was uncertain due to a previous history of rheumatic disease were excluded in the assessment. Case reports that did not present sufficient clinical data of patients were excluded. Cases that did not exclude conditions that could be confused with ReA such as rheumatoid arthritis, crystal arthropathies, and septic arthritis, cases with suspected trauma, cases that did not present the necessary laboratory and radiological examinations and cases where information was not provided about treatment and follow-up processes were excluded in the evaluation. The titles, abstracts, and full texts of the case reports were independently reviewed by two authors (BFK and AA) in terms of the aforementioned criteria. In case of disagreement between the two authors, the papers were reevaluated and the final decision was made. After implementing these inclusion and exclusion criteria, 20 papers and 21 cases were identified for evaluation. Papers published before July 12, 2021 were reviewed.
Results
A total of 21 cases from 20 papers and the current case were recorded, and the main characteristics of the cases are presented in Table 1 [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The median age of the cases was 50 (21–73) years. The ratio of male cases to females was 7/4. The most frequent comorbidities were hypertension (n = 5) and diabetes mellitus (n = 3).
The diagnosis of COVID-19 was confirmed using nasopharyngeal swab tests in 20 cases and using serological methods in 2. Hospitalization due to COVID-19 was reported in 9 cases and there was no hospitalization in 12 cases (in one case, no information was available on this subject). Additionally, intensive care unit (ICU) admission was reported in 3 cases. The median duration between diagnosis of COVID-19 and ReA was 18 (7–90) days.
Axial involvement was detected in 2 cases, monoarthritis in 10 cases, oligoarthritis in 5 cases, and polyarthritis in 5 cases. The affected joints are presented in Table 2. Enthesitis was reported in one case and there were no cases with dactylitis. Extra-articular signs were present in 2 cases as balanitis and skin lesions.
RF concentration was not reported in 5 cases, it was above the determined range in one case, and within normal limits in 16 cases. The ACPA level was not evaluated in 8 cases and was within the normal range in 14 cases. A high ACPA level was not determined in any case. ANA was not evaluated in 7 cases, it was positive in one case, and within the normal range in 14 cases. HLA-B27 was not evaluated in 11 cases, and in the remaining 11 cases, 4 were HLA-B27 positive and 7 were negative.
Synovial fluid culture was evaluated in 5 cases and was negative. Crystals in synovial fluid were investigated in 7 cases and were negative. Synovial real-time polymerase chain reaction for SARS-CoV-2 was assessed in 5 cases and was negative.
Radiological involvement (with X-Ray, ultrasonography, computed tomography, or magnetic resonance imaging) was not assessed in 5 cases. Radiological involvement was detected in 12 cases and not detected in 5 cases. C-reactive protein (CRP) data were not available in 5 cases. In the remaining cases, it was reported as high in 5 cases and the normal range in 11 cases.
In 18 cases, non-steroidal anti-inflammatory drugs were administered alone (n = 5) or along with other drugs (n = 13) to treat ReA. In 14 cases, steroids were used alone (n = 3) or along with other drugs (n = 11). Sulfasalazine was administered in one case and certolizumab was used in another case. In one case, the ReA treatment was not specified.
Discussion
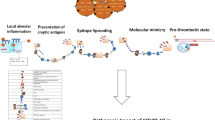
Various viral agents may influence the emergence of different autoimmune disorders in individuals with immune system vulnerability [35]. In previous viral outbreaks such as influenza, Zika, and Ebola, clinical pictures have been reported of Guillain-Barré syndrome, transverse myelitis, type 1 diabetes, vasculitis, antiphospholipid syndrome, and arthritis [36]. Although different mechanisms have been suggested in the etiopathogenesis of virus-induced arthritis, molecular mimicry has been the most emphasized [26, 36]. Molecular mimicry triggers humoral and cellular autoreactivity in the host at the end of the process by which the epitope cross-reacts between a viral agent and the host [37]. SARS-CoV-2 proteins demonstrated one minimum match to human protein in a peptidome comparison covering 37 viral proteins [38]. The stated similarities may lead to intolerance of the body’s own peptides and induce various autoimmune disorders and clinical arthritis.
ReA is diagnosed more frequently in young adults and it has been revealed that cases are concentrated between the ages of 18 and 40 years [39]. The median age of ReA cases after COVID-19 was seen to be 50 years and was considered to be slightly higher than the literature data. Asymptomatic COVID-19 patients often consist of a young population. Therefore, young adults may have been diagnosed relatively less and the median age may have increased. The incidence of ReA is high in the male population [40, 41], and post-COVID-19 ReA cases also support this data.
Less than half of the patients had been hospitalized for COVID-19, and only three patients had a history of ICU treatment. This result supports the view that there is no link between the clinical severity of COVID-19 and the development of ReA.
Considering the worldwide frequency of COVID-19 and the size of the affected population, the number of reported COVID-19-related ReA cases is low. When the reported cases are examined, the rate of cases with enthesitis, dactylitis, and extra-articular manifestations is lower than the literature data [42]. The use of hydroxychloroquine and corticosteroids for treating SARS-CoV-2 infection, which has the potential to prevent arthritis, enthesitis, dactylitis, and extra-articular manifestations, may explain these results. Although hydroxychloroquine is ineffective for treating COVID-19, it was preferred in the early stages of the pandemic [43].
The duration between COVID-19 diagnosis and ReA varied between cases. However, it was seen that arthritis generally appeared after acute infection and during the recovery period (median: 18 days). Clinical data of ReA indicate that oligoarticular involvement predominates, followed by monoarthritis with prominent involvement of the lower extremity joints [40, 44]. Monoarthritis was the most common form of involvement in ReA cases after COVID-19, followed by oligoarthritis and polyarthritis at equal rates. The most commonly involved joints were the knee, ankle, and proximal interphalangeal joint. Lahu et al. [40] reported the most commonly involved joints in ReA to be the knee, talocrural (ankle), and metatarsophalangeal joints, which were consistent with the current results. The presence of polyarticular involvement with the involvement of the hand joints in ReA cases after COVID-19 is compatible with the clinical status of other viral-associated arthritises, which occur with a polyarticular pattern similar to rheumatoid arthritis [45].
HLA-B27 positivity has been reported at different rates in ReA. Although a study that presented HLA-B27 positivity below 10% and claimed that there was no difference from the control group [46], there are also studies reporting it as 36% and 50–80% [47, 48]. There was positivity in 4 of 11 (36.4%) post-COVID-19 ReA cases whose HLA-B27 status was evaluated in the literature. HLA-B27 positivity is not necessary for developing ReA, but it has a potentiating effect. The presence of HLA-B27 acts by presenting antigenic parts to T cells, changing the self-tolerance of the immune system, increasing the level of tumor necrosis factor-alpha, and prolonging the life cycle of micro-organisms in the host [49].
Virus-induced arthritis should be considered a diagnosis of exclusion, and extensive testing should be performed to rule out other possible diagnoses. Synovial fluid analyses were not performed in a substantial proportion of cases. Additionally, the presence of cases unevaluated in terms of RF, ACPA, and ANA reveals incomplete diagnostic processes.
Most reported post-COVID-19 ReA cases had a complete and rapid response to non-steroidal anti-inflammatory drugs, glucocorticoids, or a combination of the two. This suggests that post-COVID-19 ReA cases have a mild course and are not resistant to treatment.
Conclusion
The world is currently trying to fully recognize the consequences of SARS-CoV-2 infection and its effects on individuals. Since the early days of the pandemic, great advances have been made in gaining a more accurate information about viral pathogenicity, but there are still many issues to be explored in this area. From the beginning of the pandemic, an increasing number of cases of COVID-19-related arthritis have been reported, so the need has arisen for researchers to focus more on this area. This paper provides a review of the general characteristics of COVID-19-related ReA cases published in the literature up to the specified date. To be able to differentiate ReA from diseases that may present with similar clinical pictures, comprehensive clinical and laboratory investigations, synovial fluid analysis, and close follow-up of the patient are essential.
References
Velikova T, Georgiev T (2021) SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int 41:509–518. https://doi.org/10.1007/s00296-021-04792-9
Sarzi-Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, Antinori S, Galli M (2020) COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 38:337–342
Misra DP, Agarwal V, Gasparyan AY, Zimba O (2020) Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol 39:2055–2062. https://doi.org/10.1007/s10067-020-05073-9
Ahmed S, Gasparyan AY, Zimba O (2021) Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int 41:243–256. https://doi.org/10.1007/s00296-020-04764-5
Sener AG, Afsar I (2012) Infection and autoimmune disease. Rheumatol Int 32:3331–3338. https://doi.org/10.1007/s00296-012-2451-z
Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S (2020) Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 39:2085–2094. https://doi.org/10.1007/s10067-020-05190-5
Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, RieuxLaucat F, Kernéis Terrier B (2020) Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369:718–724. https://doi.org/10.1126/science.abc6027
Zeidler H, Hudson AP (2021) Reactive arthritis update: spotlight on new and rare infectious agents implicated as pathogens. Curr Rheumatol Rep 23:53. https://doi.org/10.1007/s11926-021-01018-6
Kuuliala A, Julkunen H, Paimela L, Peltomaa R, Kautiainen H, Repo H, Leirisalo-Repo M (2013) Double-blind, randomized, placebo-controlled study of three-month treatment with the combination of ofloxacin and roxithromycin in recent-onset reactive arthritis. Rheumatol Int 33:2723–2729. https://doi.org/10.1007/s00296-013-2794-0
Taniguchi Y, Nishikawa H, Yoshida T, Terada Y, Tada K, Tamura N, Kobayashi S (2021) Expanding the spectrum of reactive arthritis (ReA): classic ReA and infection-related arthritis including poststreptococcal ReA, Poncet’s disease, and iBCG-induced ReA. Rheumatol Int 41:1387–1398. https://doi.org/10.1007/s00296-021-04879-3
Ahmed S, Zimba O, Gasparyan AY (2021) COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol 40:2611–2619. https://doi.org/10.1007/s10067-021-05691-x
Karaarslan F, DemircioğluGüneri F, Kardeş S (2021) Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: prospective follow-up by phone interviews. Rheumatol Int 41:1263–1271. https://doi.org/10.1007/s00296-021-04882-8
Selmi C, Gershwin ME (2014) Diagnosis and classification of reactive arthritis. Autoimmun Rev 13:546–549. https://doi.org/10.1016/j.autrev.2014.01.005
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Hønge BL, Hermansen MF, Storgaard M (2021) Reactive arthritis after COVID-19. BMJ Case Rep 14:e241375. https://doi.org/10.1136/bcr-2020-241375
El Hasbani G, Jawad A, Uthman I (2021) Axial and peripheral spondyloarthritis triggered by sars-cov-2 infection: a report of two cases. Reumatismo 73:59–63. https://doi.org/10.4081/reumatismo.2021.1374
Coath FL, Mackay J, Gaffney JK (2021) Axial presentation of reactive arthritis secondary to COVID-19 infection. Rheumatology (Oxford) 60:e232–e233. https://doi.org/10.1093/rheumatology/keab009
Schenker HM, Hagen M, Simon D, Schett G, Manger B (2021) Reactive arthritis and cutaneous vasculitis after SARS-CoV-2 infection. Rheumatology (Oxford) 60:479–480. https://doi.org/10.1093/rheumatology/keaa689
Di Carlo M, Tardella M, Salaffi F (2021) Can SARS-CoV-2 induce reactive arthritis? Clin Exp Rheumatol 128(1):25–26
Saricaoglu EM, Hasanoglu I, Guner R (2021) The first reactive arthritis case associated with COVID-19. J Med Virol 93:192–193. https://doi.org/10.1002/jmv.26296
Liew IY, Mak TM, Cui L, Vasoo S, Lim XR (2020) A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol 26:233. https://doi.org/10.1097/RHU.0000000000001560
Jali I (2020) Reactive arthritis after COVID-19 infection. Cureus 12:e11761. https://doi.org/10.7759/cureus.11761
Fragata I, Mourão AF (2020) Coronavirus disease 19 (COVID-19) complicated with post-viral arthritis. Acta Reumatol Port 45:278–280
Ono K, Kishimoto M, Shimasaki T, Uchida H, Kurai D, Deshpande GA, Komagata Y, Kaname S (2020) Reactive arthritis after COVID-19 infection. RMD Open 6:e001350. https://doi.org/10.1136/rmdopen-2020-001350
De Stefano L, Rossi S, Montecucco C, Bugatti S (2020) Transient monoarthritis and psoriatic skin lesions following COVID-19. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2020-218520
Gasparotto M, Framba V, Piovella C, Doria A, Iaccarino L (2021) Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol 40:3357–3362. https://doi.org/10.1007/s10067-020-05550-1
Yokogawa N, Minematsu N, Katano H, Suzuki T (2020) Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2020-218281
Sureja NP, Nandamuri D (2021) Reactive arthritis after SARS-CoV-2 infection. Rheumatol Adv Pract 5:rkab001. https://doi.org/10.1093/rap/rkab001
Danssaert Z, Raum G, Hemtasilpa S (2020) Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus 12:e9698. https://doi.org/10.7759/cureus.9698
Saikali W, Gharib S (2021) The first non-radiographic axial spondyloarthrits with COVID-19. Immun Inflamm Dis. https://doi.org/10.1002/iid3.448
Shokraee K, Moradi S, Eftekhari T, Shajari R, Masoumi M (2021) Reactive arthritis in the right hip following COVID-19 infection: a case report. Trop Dis Travel Med Vaccines 7:18. https://doi.org/10.1186/s40794-021-00142-6
Gibson M, Sampat K, Coakley G (2020) A self-limiting symmetrical polyarthritis following COVID-19 infection. Rheumatol Adv Pract. https://doi.org/10.1093/rap/rkaa052.014
Ghauri IG, Mukarram MS, Ishaq K, Riaz SU (2020) Post COVID-19 reactive arthritis: an emerging existence in the spectrum of musculoskeletal complications of SARS-CoV-2 ınfection. J Clin Stud Med Case Rep 7:101. https://doi.org/10.24966/CSMC-8801/100101
Cincinelli G, Di Taranto R, Orsini F, Rindone A, Murgo A, Caporali R (2021) A case report of monoarthritis in a COVID-19 patient and literature review: Simple actions for complex times. Medicine (Baltimore) 100:e26089. https://doi.org/10.1097/MD.0000000000026089
Arleevskaya MI, Manukyan G, Inoue R, Aminov R (2017) Editorial: microbial and environmental factors in autoimmune and inflammatory diseases. Front Immunol 8:243. https://doi.org/10.3389/fimmu.2017.00243
Shah S, Danda D, Kavadichanda C, Das S, Adarsh MB, Negi VS (2020) Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol Int 40:1539–1554. https://doi.org/10.1007/s00296-020-04639-9
Kim B, Kaistha SD, Rouse BT (2006) Viruses and autoimmunity. Autoimmunity 39:71–77. https://doi.org/10.1080/08916930500484708
Lyons-Weiler J (2020) Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoimmun 3:100051. https://doi.org/10.1016/j.jtauto.2020.100051
Bentaleb I, Abdelghani KB, Rostom S, Amine B, Laatar A, Bahiri R (2020) Reactive arthritis: update. Curr Clin Microbiol Rep. https://doi.org/10.1007/s40588-020-00152-6
Lahu A, Backa T, Ismaili J, Lahu V, Saiti V (2015) Modes of presentation of reactive arthritis based on the affected joints. Med Arch 69:42–45. https://doi.org/10.5455/medarh.2015.69.42-45
Kim PS, Klausmeier TL, Orr DP. Reactive arthritis: a review. J Adolesc Health 44:309–315. https://doi.org/10.1016/j.jadohealth.2008.12.007
Stavropoulos PG, Soura E, Kanelleas A, Katsambas A, Antoniou C (2015) Reactive arthritis. J Eur Acad Dermatol Venereol 29:415–424. https://doi.org/10.1111/jdv.12741
Ibáñez S, Martínez O, Valenzuela F, Silva F, Valenzuela O (2020) Hydroxychloroquine and chloroquine in COVID-19: should they be used as standard therapy? Clin Rheumatol 39:2461–2465. https://doi.org/10.1007/s10067-020-05202-4
Ozgül A, Dede I, Taskaynatan MA, Aydogan H, Kalyon TA (2006) Clinical presentations of chlamydial and non-chlamydial reactive arthritis. Rheumatol Int 26:879–885. https://doi.org/10.1007/s00296-005-0094-z
Marks M, Marks JL (2016) Viral arthritis. Clin Med (Lond) 16:129–134. https://doi.org/10.7861/clinmedicine.16-2-129
Garcia Ferrer HR, Azan A, Iraheta I, Von Feldt J, Espinoza LR, Manasson J, Scher JU, Garcia Kutzbach A, Ogdie A (2018) Potential risk factors for reactive arthritis and persistence of symptoms at 2 years: a case-control study with longitudinal follow-up. Clin Rheumatol 37:415–422. https://doi.org/10.1007/s10067-017-3911-3
Hannu T, Mattila L, Siitonen A, Leirisalo-Repo M (2005) Reactive arthritis attributable to Shigella infection: a clinical and epidemiological nationwide study. Ann Rheum Dis 64:594–598. https://doi.org/10.1136/ard.2004.027524
Sieper J. Pathogenesis of reactive arthritis. Curr Rheumatol Rep 3:412–418. https://doi.org/10.1007/s11926-996-0012-8
Protopopov M, Sieper J, Haibel H, Listing J, Rudwaleit M, Poddubnyy D (2017) Relevance of structural damage in the sacroiliac joints for the functional status and spinal mobility in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Res Ther 19:240. https://doi.org/10.1186/s13075-017-1453-3
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
BFK and AA designed the study. BFK and AA reviewed the literature and collected the data. BFK analyzed the data. MSA and BFK interpreted the data analyses. BFK and AA drafted the study and reviewed it critically for important intellectual content. BFK and AA prepared tables and figures. BFK and AA approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Informed consent
The presented patient provided signed consent for the publication of this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kocyigit, B.F., Akyol, A. Reactive arthritis after COVID-19: a case-based review. Rheumatol Int 41, 2031–2039 (2021). https://doi.org/10.1007/s00296-021-04998-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-021-04998-x