Abstract
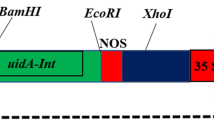
An efficient protocol for the production of transgenic Brassica napus cv. Westar plants was developed by optimizing two important parameters: preconditioning time and co-cultivation time. Agrobacterium tumefaciens-mediated transformation was performed using hypocotyls as explant tissue. Two variants of a green fluorescent protein (GFP)-encoding gene—mGFP5-ER and eGFP—both under the constitutive expression of the cauliflower mosaic virus 35S promoter, were used for the experiments. Optimizing the preconditioning time to 72 h and co-cultivation time with Agrobacterium to 48 h provided the increase in the transformation efficiency from a baseline of 4% to 25%. With mGFP5-ER, the transformation rate was 17% and with eGFP it was 25%. Transgenic shoots were selected on 200 mg/l kanamycin. Rooting efficiency was 100% on half-strength Murashige and Skoog medium with 10 g/l sucrose and 0.5 mg/l indole butyric acid in the presence of kanamycin.




Similar content being viewed by others
References
Altenbach SB, Kuo C-C, Staraci LC, Pearson KW, Wainwright C, Georgescu A, Townsend J (1992) Accumulation of a Brazil nut albumin in seeds of transgenic canola results in enhanced levels of seed protein methionine. Plant Mol Biol 18:235–246
Binns AN (1991) Transformation of wall deficient cultured tobacco protoplasts by Agrobacterium tumefaciens. Plant Physiol 96:498–506
Choi PS, Soh WY, Kim YS, Yoo OJ, Liu JR (1994) Genetic transformation and plant regeneration of water-melon using Agrobacterium tumefaciens. Plant Cell Rep 13:344–348
Confalonieri M, Balestrazzi A, Bisoffi S (1994) Genetic transformation of Populus nigra by Agrobacterium tumefaciens. Plant Cell Rep13:256–261
Debergh P, Harbaoui Y, Lemeur R (1981) Mass propagation of globe artichoke (Cynara scolymus): evaluation of different hypotheses to overcome vitrification with special reference to water potential. Physiol Plant 53:181–187
De Block M, De Brower D, Tenning P (1989) Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol 91:694–701
Figueiredo SFL, Albarello N, Viana VRC (2001) Micropropagation of Rollinia mucosa (Jacq). Baill. In Vitro Cell Dev Biol Plant 37:471–475
Fry J, Barnason A, Horsch RB (1987) Transformation of Brassica napus with Agrobacterium based vectors. Plant Cell Rep 6:321–325
George EF (1996) Plant propagation by tissue culture, part 2. The technology. Exegetics, Edington Wilts
Halfhill MD, Richards HA, Mabon SA, Stewart CN Jr (2001) Expression of GFP and Bt transgenes in Brassica napus and hybridization with Brassica rapa. Theor Appl Genet 103:659–667
Harper BK, Mabon SA, Leffel SM, Halfhill MD, Richards HA, Moyer KA, Stewart CN Jr (1999) Green fluorescent protein in transgenic plants indicates the presence and expression of a second gene. Nat Biotechnol 17:1125–1129
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Jacq B, Lesobre O, Sangwan RS, Sangwan-Norreel BS (1993) Factors influencing T-DNA transfer in Agrobacterium-mediated transformation in sugarbeet. Plant Cell Rep 12:621–624
Janssens J, Sonville AD, Moens T, Opsomer C (1995) Genetic modification of Brassica napus by Agrobacterium-mediated transformation. In: Workshop on Brassica napus L. Book of abstracts, L4. Eucarpia, Helsingør
Knutzon DS, Thompson GA, Radke SE, Johnson WB, Knauf VC, Kridl JC (1992) Modification of Brassica seed oil by antisense expression of a stearoyl-acyl carrier protein desaturase gene. Proc Natl Acad Sci USA 89:2624–2628
Kooi LT, Keng CL, Hoe CTK (1999) In vitro rooting of sentang shoots (Azadirachta excelsa L.) and acclimatization of the plantlets. In Vitro Cell Dev Biol Plant 35:396–400
Mehra-Palta A, Graves A, Kraemer M, Hill K, Eagan N (1991) Genetic transformation of Brassica napus and Brassica rapa. In: McGregor DI (ed) Proc 8th GCIRC Congr. University Extension Press, Saskatoon, Sask., Canada, pp 1108–1115
Moloney MM, Walker JM, Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8:238–242
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Ovesná J, Ptacek L, Opartny Z (1993) Factors influencing the regeneration capacity of oilseed rape and cauliflower in transformation experiments. Biol Plant 35:107–112
Owens LD, Smigocki AC (1988) Transformation of soybean cells using mixed strains of Agrobacterium tumefaciens and phenolic compounds. Plant Physiol 88:570–573
Poulsen GB (1996) Genetic transformation of Brassica. Plant Breed 115:209–225
Pratt LH, McCurdy DW, Shimazaki Y, Cordonnier MM (1986) Immunodetection of phytochrome: immunocytochemistry, immunoblotting, and immunoquantitation. Modern methods of plant analysis. Springer, Berlin Heidelberg New York
Pua EC, Mehra Palta A, Nagy F, Chua NH (1987) Transgenic plants of Brassica napus L. Biotechnology 5:815–817
Radke SE, Andrews BM, Moloney MM, Crouch ML, Krid JC, Knauf VC (1988) Transformation of Brassica napus L. using Agrobacterium tumefaciens: developmentally regulated expression of a reintroduced napin gene. Theor Appl Genet 75:685–694
Samantaray S, Rout GR, Das P (1995) An in vitro study on organogenesis in Trema orientalis (Blume) Linn. Plant Sci 105:87–94
Sangwan RS, Bourgeois Y, Brown S, Vasseur G, Sangwan-Norreel BS (1992) Characterization of competent cells and early events of Agrobacterium-mediated genetic transformation of Arabidopsis thaliana. Planta 188:439–456
Schmidt R, Willmitzer L (1985) High frequency Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana leaf and cotyledon explants. Plant Cell Rep 7:583–586
Sheikholeslam SN, Weeks DP (1987) Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol Biol 8:291–298
Sovero M (1993) Rapeseed, a new oilseed crop for the United States. In: Janick J, Simon JE (eds) New crops. Wiley, New York, pp 302–307
Spencer PA, Towers GHN (1991) Restricted occurrence of acetophenone signal compounds. Phytochemistry 30:2933–2937
Stachel SE, Messens M, Van Montagu M, Zambryski P (1985) Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Stewart CN Jr (1997) Rapid DNA extraction from plants. In: Micheli MR, Bova R (eds) Fingerprinting methods based on arbitrarily primed PCR. Springer, Berlin Heidelberg New York, pp 25–28
Stewart CN Jr (2001) The utility of green fluorescent protein in transgenic plants. Plant Cell Rep 20:376–382
Stewart CN Jr, Adang MJ, All JA, Raymer PL, Ramachandran S, Parrott WA (1996) Insect control and dosage effects in transgenic canola containing a synthetic Bacillus thuringiensis cryIAC gene. Plant Physiol 112:115–120
Sunilkumar G, Vijayachandra K, Veluthambi K (1999) Pre-incubation of cut tobacco leaf promotes Agrobacterium-mediated transformation by increasing vir gene induction. Plant Sci 141:51–58
Upreti J, Dhar U (1996) Micropropagation of Bauhinia vahlii Wight & Arnott—a leguminous liana. Plant Cell Rep 16:250–254
Von Arnold SV, Eriksson T (1984) Effect of agar concentration on growth and anatomy of adventitious shoots of Picea abies. Plant Cell Tissue Organ Cult 3:257–264
Zhang FL, Takahata Y, Watanabe M, Xu JB (2000) Agrobacterium-mediated transformation of cotyledonary explants of Chinese cabbage (Brassica campestris L. ssp. pekinensis). Plant Cell Rep 19:569–575
Ziv M, Meit G, Halevy A (1983) Factors influencing the production of hardened carnation plantlets in vitro. Plant Cell Tissue Organ Cult 2:55−60
Acknowledgements
We thank Jim Haseloff for providing the mGFP5-ER construct, Steve Mabon for cloning eGFP, Brenda Kivela for assisting in this project and all our laboratory colleagues for their support. We thank the NASA HEDS program for funding this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.C. Jordan
Rights and permissions
About this article
Cite this article
Cardoza, V., Stewart, C.N. Increased Agrobacterium-mediated transformation and rooting efficiencies in canola (Brassica napus L.) from hypocotyl segment explants. Plant Cell Rep 21, 599–604 (2003). https://doi.org/10.1007/s00299-002-0560-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-002-0560-y