Abstract
Key message
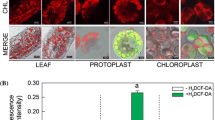
NO generation is studied in the protoplast chloroplasts. NO, ONOO − and ROS (O − 2 and H 2 O 2 ) are generated in chloroplasts. Nitric oxide synthase-like protein appears to be involved in NO generation.
Abstract
Nitric oxide stimulates chlorophyll biosynthesis and chloroplast differentiation. The present study was conducted to better understand the process of NO generation in the leaf chloroplasts and protoplasts. NO, peroxynitrite and superoxide anion were investigated in the protoplasts and isolated chloroplasts using specific dyes, confocal laser scanning and light microscopy. The level of NO was highest after protoplast isolation and subsequently decreased during culture. Suppression of NO signal in the presence of PTIO, suggests that diaminofluorescein-2 diacetate (DAF-2DA) detected NO. Detection of peroxynitrite, a reaction product of NO and superoxide anion, further suggests NO generation. Moreover, generation of NO and peroxynitrite in the chloroplasts of wild-type Arabidopsis and their absence or weak signals in the leaf-derived protoplasts of Atnoa1 mutants confirmed the reactivity of DAF-2DA and aminophenyl fluorescein to NO and peroxynitrite, respectively. Isolated chloroplasts also showed signal of NO. Suppression of NO signal in the presence of 100 μM nitric oxide synthase inhibitors [l-NNA, Nω-nitro-l-arginine and PBIT, S,S′-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea] revealed that nitric oxide synthase-like system is involved in NO synthesis. Suppression of NO signal in the protoplasts isolated in the presence of cycloheximide suggests de novo synthesis of NO generating protein during the process of protoplast isolation. Furthermore, the lack of inhibition of NO production by sodium tungstate (250 μM) and inhibition by l-NNA, and PBIT suggest involvement NOS-like protein, but not nitrate reductase, in NO generation in the leaf chloroplasts and protoplasts.











Similar content being viewed by others
Abbreviations
- APF:
-
Aminophenyl fluorescein
- DAF-2DA:
-
Diaminofluorescein-2 diacetate
- H2DCF-DA:
-
2,7-Dichlorodihydrofluorescein diacetate
- l-NNA:
-
Nω-nitro-l-arginine
- NO:
-
Nitric oxide
- NOS:
-
NO synthase
- PBIT:
-
S,S′-1,3-Phenylene-bis(1,2-ethanediyl)-bis-isothiourea
- PCD:
-
Programmed cell death
- PTIO:
-
2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide
- ROS:
-
Reactive oxygen species
References
Arita NO, Cohen MF, Tokuda G, Yamasaki H (2006) Fluorometric detection of nitric oxide with diaminofluoresceins (DAFs): applications and limitations of plant NO research. In: Lamattina L, Polacco JC (eds) Nitric oxide in plant growth, development and stress physiology, Plant Cell Monographs, vol 6. Springer, Berlin, pp 269–280
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Chaki M, Valderrama R, Fernández-Ocaña AM, Carreras A, Gómez-Rodríguez MV, López-Jaramillo J, Begara-Morales JC, Sánchez-Calvo B, Luque F, Leterrier M, Corpas FJ, Barroso JB (2011) High temperature triggers the metabolism of S-nitrosothiols in sunflower mediating a process of nitrosative stress which provokes the inhibition of ferredoxin-NADP reductase by tyrosine nitration. Plant Cell Environ 34:1803–1818
Corpas FJ, Barroso JB, Carreras A, Quirós M, León AM, Romero-Puertas MC, Esteban FJ, Valderrama R, Palma JM, Sandalio LM, Gomez M, del Rio LA (2004) Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol 136:2722–2733
Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Río LA (2006) Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 224:246–254
Corpas FJ, Palma JM, del Río LA, Barroso JB (2009) Evidence supporting the existence of l-arginine dependent nitric oxide synthase activity in plants. New Phytol 184:9–14
Crawford NM, Galli M, Tischner R, Heimer YM, Okamoto M, Mack A (2006) Response to Zemojtel et al.: plant nitric oxide synthase: back to square one. Trends Plant Sci 11:526–527
del Río LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plant. Phytochemistry 65:783–792
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Desikan R, Cheung M-K, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot 55:205–212
Faraco M, Di Sansebastiano GP, Spelt K, Koes RE, Quattrocchio FM (2011) One protoplast is not the other! Plant Physiol 156:474–478
Gas E, Flores-Pérez U, Sauret-Güeto S, Rodríguez-Concepción M (2009) Hunting for plant nitric oxide synthase provides new evidence of a central role for plastids in nitric oxide metabolism. Plant Cell 21:18–23
Gaupels F, Spiazzi-Vandelle E, Yang D, Delledonne M (2011) Detection of peroxynitrite accumulation in Arabidopsis thaliana during the hypersensitive defense response. Nitric Oxide 25:222–228
Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R (2000) Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275:7757–7763
Graziano M, Lamattina L (2005) Nitric oxide and iron in plants: an emerging and converging story. Trends Plant Sci 10:4–8
Graziano M, Beligni MV, Lamattina L (2002) Nitric oxide improves internal iron availability in plants. Plant Physiol 130:1852–1859
Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17:3436–3450
Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signalling. Science 302:100–103
Gupta KJ, Kaiser WM (2010) Production and scavenging of nitric oxide by barley root mitochondria. Plant Cell Physiol 51:576–584
Hope BT, Michael GJ, Knigge KM, Vincent SR (1991) Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Nat Acad Sci USA 88:2811–2814
Jasid S, Simontacchi M, Bartoli CG, Puntarulo S (2006) Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol 142:1246–1255
Kieselbach T, Hagman Å, Andersson B, Schröder WP (1998) The thylakoid lumen of chloroplasts: isolation and characterization. J Biol Chem 273:6710–6716
Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T (1998) Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70:2446–2453
Kumar P, Tewari RK, Sharma PN (2010) Sodium nitroprusside-mediated alleviation of iron deficiency and modulation of antioxidant responses in maize plants. AoB Plants 2010:plq002
Mulo P, Pursiheimo S, Hou CX, Tyystjärvi T, Aro EM (2003) Multiple effects of antibiotics on chloroplast and nuclear gene expression. Func Plant Biol 30:1097–1103
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T (1998) Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett 427:263–266
Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128:13–16
Padmaja S, Huie RE (1993) The reaction of nitric oxide with organic peroxylo radicals. Biochem Biophys Res Commun 195:539–544
Ree KV, Gehl B, Chehab EW, Tsai YC, Braam J (2011) Nitric oxide accumulation in Arabidopsis is independent of NOA1 in the presence of sucrose. Plant J 68:225–233
Ribeiro EA Jr, Cunha FQ, Tamashiro WM, Martins IS (1999) Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett 445:283–286
Robinson JM (1986) Carbon dioxide and nitrite photoassimilatory processes do not intercompete for reducing equivalents in spinach, and soybean leaf chloroplasts. Plant Physiol 80:676–684
Rodriguez-Serrano M, Barany I, Prem D, Coronado MJ, Risueno MC, Testillano PS (2012) NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot 63:2007–2024
Saito S, Yamamoto-Katou A, Yoshioka H, Doke N, Kawakita K (2006) Peroxynitrite generation and tyrosine nitration in defense responses in tobacco BY-2 cells. Plant Cell Physiol 47:689–697
Simontacchi M, Jasid S, Puntarulo S (2004) Nitric oxide generation during early germination of sorghum seeds. Plant Sci 167:839–847
Swanson SJ, Choi WG, Chanoca A, Gilroy S (2011) In Vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu Rev Plant Biol 62:273–297
Tewari RK, Paek KY (2011) Salicylic acid-induced nitric oxide and ROS generation stimulate ginsenoside accumulation in Panax ginseng roots. J Plant Growth Reg 30:396–404
Tewari RK, Kumar P, Sharma PN (2006) Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta 223:1145–1153
Tewari RK, Hahn EJ, Paek KY (2008a) Function of nitric oxide and superoxide anion in the adventitious root development and antioxidant defence in Panax ginseng. Plant Cell Rep 27:563–573
Tewari RK, Hahn EJ, Paek KY (2008b) Modulation of copper toxicity-induced oxidative damage by nitric oxide supply in the adventitious roots of Panax ginseng. Plant Cell Rep 27:171–181
Tewari RK, Kumar P, Kim S, Hahn EJ, Paek KY (2009) Nitric oxide retards xanthine oxidase-mediated superoxide anion generation in Phalaenopsis flower: an implication of NO in the senescence and oxidative stress regulation. Plant Cell Rep 28:267–279
Tewari RK, Watanabe D, Watanabe M (2012) Chloroplastic NADPH oxidase-like activity-mediated perpetual hydrogen peroxide generation in the chloroplast induces apoptotic-like death of Brassica napus leaf protoplasts. Planta 235:99–110
Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99:517–522
Vanin AF, Svistunenko DA, Mikoyan VD, Serezhenkov VA, Fryer MJ, Baker NR, Cooper CE (2004) Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. J Biol Chem 279:24100–24107
Watanabe M, Watanabe Y, Shimada N (1992) Colorimetric estimation of leaf-protoplast potential-to-divide by use of 2,3,5-triphenyl tetrazolium chloride. J Plant Physiol 44:111–114
Watanabe M, Kawasaki H, Itho Y, Watanabe Y (1998) Senescence development of Brassica napus leaf protoplasts during isolation and subsequent culture. J Plant Physiol 152:487–493
Watanabe M, Setoguchi D, Uehara K, Ohtsuka W, Watanabe Y (2002a) Apoptotic-like cell death of Brassica napus leaf protoplasts. New Phytol 156:417–426
Watanabe M, Suzuki K, Kawasaki H, Watanabe Y (2002b) Differential responses of Brassica napus and Petunia hybrida to leaf protoplast isolation stress. Physiol Plant 114:645–651
Xiong J, Fu G, Yang Y, Zhu C, Tao L (2012) Tungstate: is it really a specific nitrate reductase inhibitor in plant nitric oxide research? J Exp Bot 63:33–41
Yasuda K, Watanabe Y, Watanabe M (2007) Generation of intracellular reactive oxygen species during the isolation of Brassica napus leaf protoplasts. Plant Biotechnol 24:361–366
Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91(phox) homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15:706–718
Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inzé D, Delledonne M, Van Breusegem F (2006) Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol 141:404–411
Zhang L, Wang Y, Zhao L, Shi S, Zhang L (2006) Involvement of nitric oxide in light-mediated greening of barley seedlings. J Plant Physiol 163:818–826
Zhao DY, Tian QY, Li LH, Zhang WH (2007a) Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot 100:497–503
Zhao MG, Tian QY, Zhang WH (2007b) Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol 144:206–217
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Acknowledgments
This work was financially supported by Japan Society for the Promotion of Science (JSPS) as a postdoctoral fellowship (P08413) to RKT and grant-in-aid for scientific research. Authors are thankful to Prof. Eisho Nishino and Dr. Kohei Mishina, Chiba University, for their help in preparation of microtome sections of leaf and confocal microscopy of samples, respectively.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by P. Kumar.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tewari, R.K., Prommer, J. & Watanabe, M. Endogenous nitric oxide generation in protoplast chloroplasts. Plant Cell Rep 32, 31–44 (2013). https://doi.org/10.1007/s00299-012-1338-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1338-5