Abstract
Key message
Overexpression of Withania somnifera SGT gene (WssgtL3.1) in transgenic Arabidopsis improves various agronomic and physiological traits and alters conjugated sterol levels to mitigate the effect of salt stress.
Abstract
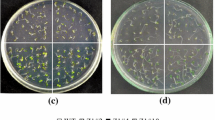
Sterols are essential constituents of cell membranes that are involved in several biological functions, including response to various biotic and abiotic stresses by altering membrane permeability and signaling pathways. Sterol glycosyltransferases (SGTs) are enzymes that are involved in sterol modification by converting sterols into sterol-conjugates to play essential roles in adaptive responses. However, their roles under abiotic stresses are lesser-known. Among abiotic stresses, salinity imposes serious threat to crop yield worldwide, hence the present study intends to investigate the role of WssgtL3.1-overexpressed Arabidopsis plants under salt stress indicating the crosstalk between SGT gene and salinity to develop improved crop varieties with better stress tolerance ability. The findings revealed that overexpression of WssgtL3.1 gene in A. thaliana improved the resistance against salt stress in the overexpressing lines. Transgenic lines showed significantly higher germination rate, increased plant growth with less chlorophyll damage compared to wild-type (WT) control plants. Moreover, better tolerance also correlated with enhanced osmolytes (proline and soluble sugar), better membrane integrity, decreased H2O2 production and lesser MDA accumulation and Na+/K+ ratio with more negative osmotic potential in overexpressed lines. Additionally, in sterol profiling, significant enhancement in stigmasterol was also observed in transgenic lines than WT plants. Furthermore, in expression profiling, salt responsive genes LEA 4–5, sucrose synthase, and transporter of monosaccharide (ERD) significantly upregulated in overexpressing lines as compared to WT. Thus our data strongly support the defensive role of Withania somnifera SGT gene (WssgtL3.1) against salt stress and contribute to improved salinity tolerance in plants through sterol modulation.







Similar content being viewed by others
References
Aboobucker SI, Suza WP (2019) Why do plants convert sitosterol to stigmasterol? Front Plant Sci 10:354
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta Vulgaris. Plant Physiol 24:1–15
Bowles D, Lim EK, Poppenberger B, Vaistij FE (2006) Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 57:567–597. https://doi.org/10.1146/annurev.arplant.57.032905.105429
Brini F et al (2007) Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. Plant Cell Rep 26:2017–2026
Busch F, Huner NPA, Ensminger I (2009) Biochemical constrains limit the potential of the photochemical reflectance index as a predictor of effective quantum efficiency of photosynthesis during the winter spring transition in Jack pine seedlings. Funct Plant Biol 36:1016–1026. https://doi.org/10.1071/FP08043
Carillo P, Gibon Y (2011) Protocol: extraction and determination of proline. PrometheusWiki.
Chaturvedi P, Misra P, Tuli R (2011) Sterol glycosyltransferases—the enzymes that modify sterols. Appl Biochem Biotechnol 165:47–68. https://doi.org/10.1007/s12010-011-9232-0
Chaturvedi P, Mishra M, Akhtar N, Gupta P, Mishra P, Tuli R (2012) Sterol glycosyltransferases-identification of members of gene family and their role in stress in Withania somnifera. Mol Biol Rep 39:9755–9764. https://doi.org/10.1007/s11033-012-1841-3
Chauhan PS, Lata C, Tiwari S, Chauhan AS, Mishra SK, Agrawal L, Chakrabarty D, Nautiyal CS (2019) Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci Rep 9(1):1–3
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cooke DT, Burden RS (1990) Lipid modulation of plasma membrane-bound ATPases. Physiol Plant 78:153–159
DeBolt S, Scheible WR, Schrick K, Auer M, Beisson F, Bischoff V, Bouvier-Navé P, Carroll A, Hematy K, Li Y, Milne J (2009) Mutations in UDP-glucose: sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds. Plant Physiol 151(1):78–87
Deuticke B, Haest C (1987) Lipid modulation of transport proteins in vertebrate cell membranes. Annu Rev Physiol 49:221–235
El-Beltagi HS, Mohamed HI (2013) Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Not Bot Horti Agrobo 41:44–57
Ferrer A, Altabella T, Arró M, Boronat A (2017) Emerging roles for conjugated sterols in plants. Prog Lipid Res 67:27–37
Grille S, Zaslawski A, Thiele S, Plat J, Warnecke D (2010) The functions of steryl glycosides come to those who wait: recent advances in plants, fungi, bacteria and animals. Prog Lipid Res 49:262–288
Hassanein R, Hashem H, Khalil R (2012) Stigmasterol treatment increases salt stress tolerance of faba bean plants by enhancing antioxidant systems. Plant Omics 5:476
Hayford MB, Medford JI, Hoffman NL, Rogers SG, Klee HJ (1988) Development of a plant transformation selection system based on expression of genes encoding gentamicin acetyltransferases. Plant physiol 86:1216–1222
Huang X, Wang G, Shen Y, Huang Z (2012) The wheat gene TaST can increase the salt tolerance of transgenic Arabidopsis. Plant Cell Rep 31:339–347
Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45:346–350
Lara JA, Burciaga-Monge A, Chávez A, Revés M, Lavilla R, Arró M, Boronat A, Altabella T, Ferrer A (2018) Identification and characterization of sterol acyltransferases responsible for steryl ester biosynthesis in tomato. Front Plant Sci 9:588
Li W, Li M, Zhang W, Welti R, Wang X (2004) The plasma membrane–bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nat Biotechnol 22:427–433
Li Y, He N, Hou J, Xu L, Liu C, Zhang J, Wang Q, Zhang X, Wu X (2018) Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front Ecol Evol 6:64
Lim EK, Bowles DJ (2004) A class of plant glycosyltransferases involved in cellular homeostasis. EMBO J 23:2915–2922. https://doi.org/10.1038/sj.emboj.76002957600295
Liu W-H, Ding B, Ruan X-M, Xu H-T, Yang J, Liu S-M (2007) Analysis of free and conjugated phytosterols in tobacco by an improved method using gas chromatography–flame ionization detection.J. Chromatogr A 1163:304–311
Madina BR, Sharma LK, Chaturvedi P, Sangwan RS, Tuli R (2007) Purification and characterization of a novel glucosyltransferase specific to 27beta-hydroxy steroidal lactones from Withania somnifera and its role in stress responses. Biochim Biophys Acta 1774:1199–1207. https://doi.org/10.1016/j.bbapap.2007.06.015
Mishra MK, Chaturvedi P, Singh R, Singh G, Sharma LK, Pandey V, Kumari N, Misra P (2013) Overexpression of WsSGTL1 gene of Withania somnifera enhances salt tolerance, heat tolerance and cold acclimation ability in transgenic Arabidopsis plants. PLoS ONE 8(4):e63064
Mishra MK, Singh G, Tiwari S, Singh R, Kumari N, Misra P (2015) Characterization of Arabidopsis sterol glycosyltransferase TTG15/UGT80B1 role during freeze and heat stress. Plant Signal Behav 10:e1075682. https://doi.org/10.1080/15592324.2015.1075682
Mishra MK, Srivastava M, Singh G, Tiwari S, Niranjan A, Kumari N, Misra P (2017) Overexpression of Withania somnifera SGTL1 gene resists the interaction of fungus Alternaria brassicicola in Arabidopsis thaliana. Physiol Mol Plant Pathol 97:11–19
Pandey V, Niranjan A, Atri N, Chandrashekhar K, Mishra MK, Trivedi PK, Misra P (2014) WsSGTL1 gene from Withania somnifera, modulates glycosylation profile, antioxidant system and confers biotic and salt stress tolerance in transgenic tobacco. Planta 239:1217–1231. https://doi.org/10.1007/s00425-014-2046-x
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Ramirez-Estrada K, Castillo N, Lara JA, Arró M, Boronat A, Ferrer A, Altabella T (2017) Tomato UDP-glucose sterol glycosyltransferases: a family of developmental and stress regulated genes that encode cytosolic and membrane-associated forms of the enzyme Front. Plant Sci 8:984
Rogowska A, Szakiel A (2020) The role of sterols in plant response to abiotic stress. Phytochem Rev 19:1525–1538
Rudell DR, Buchanan DA, Leisso RS, Whitaker BD, Mattheis JP, Zhu Y, Varanasi V (2011) Ripening, storage temperature, ethylene action, and oxidative stress alter apple peel phytosterol metabolism. Phytochemistry 72:1328–1340
Saema S, Rahman LU, Singh R, Niranjan A, Ahmad IZ, Misra P (2016) Ectopic overexpression of WsSGTL1, a sterol glucosyltransferase gene in Withania somnifera, promotes growth, enhances glycowithanolide and provides tolerance to abiotic and biotic stresses. Plant Cell Rep 35:195–211. https://doi.org/10.1007/s00299-015-1879-510.1007/s00299-015-1879-5
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. Chlorophyll a fluorescence. Springer, Berlin, pp 279–319
Sharma LK, Madina BR, Chaturvedi P, Sangwan RS, Tuli R (2007) Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family in Withania somnifera. Arch Biochem Biophys 460:48–55. https://doi.org/10.1016/j.abb.2007.01.024
Singh R, Pandey N, Naskar J, Shirke PA (2015) Physiological performance and differential expression profiling of genes associated with drought tolerance in contrasting varieties of two Gossypium species. Protoplasma 252:423–438
Singh G, Tiwari M, Singh SP, Singh S, Trivedi PK, Misra P (2016) Silencing of sterol glycosyltransferases modulates the withanolide biosynthesis and leads to compromised basal immunity of Withania somnifera. Sci Rep 6:25562. https://doi.org/10.1038/srep25562
Stein O, Granot D (2019) An overview of sucrose synthases in plants. Front Plant Sci 10:95
Summermatter K, Sticher L, Métraux J-P (1995) Systemic responses in Arabidopsis thaliana infected and challenged with Pseudomonas syringae pv. syringae. Plant Physiol 108:1379–1385
Sun Y-G, Wang B, Jin S-H, Qu X-X, Li Y-J, Hou B-K (2013) Ectopic expression of Arabidopsis glycosyltransferase UGT85A5 enhances salt stress tolerance in tobacco. PLoS ONE 8:e59924
Surjus A, Durand M (1996) Lipid changes in soybean root membranes in response to salt treatment. J Exp Bot 47:17–23. https://doi.org/10.1093/Jxb/47.1.17
Tapken W, Murphy AS (2015) Membrane nanodomains in plants: capturing form, function, and movement. J Exp Bot 66:1573–1586
Tiwari S, Lata C, Chauhan PS, Nautiyal CS (2016) Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99:108–117. https://doi.org/10.1016/j.plaphy.2015.11.001
Tiwari S, Prasad V, Chauhan PS, Lata C (2017) Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front Plant Sci 8:1510
Tiwari S, Gupta SC, Chauhan PS, Lata C (2020) An OsNAM gene plays important role in root rhizobacteria interaction in transgenic Arabidopsis through abiotic stress and phytohormone crosstalk. Plant cell rep. https://doi.org/10.1007/s00299-020-02620-1
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139
Zhang H, Mao X, Wang C, Jing R (2010) Overexpression of a common wheat gene TaSnRK2. 8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS ONE 5:e16041
Acknowledgments
The Director, Council of Scientific and Industrial Research-National Botanical Research Institute, is gratefully acknowledged by authors for providing the facilities. ST acknowledges CSIR-SRF for providing financial support to carry out the research work.
Author information
Authors and Affiliations
Contributions
MKM conceived and designed research. MKM and ST conducted experiments and analyzed data. MKM and ST wrote the manuscript. PM improvised the language of manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Aryadeep Roychoudhury.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mishra, M.K., Tiwari, S. & Misra, P. Overexpression of WssgtL3.1 gene from Withania somnifera confers salt stress tolerance in Arabidopsis. Plant Cell Rep 40, 2191–2204 (2021). https://doi.org/10.1007/s00299-021-02666-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-021-02666-9