Abstract
Objectives
Sarcopenia and changes in body composition following neoadjuvant chemotherapy (NAC) may affect clinical outcome. We assessed the associations between CT body composition changes following NAC and outcomes in oesophageal cancer.
Methods
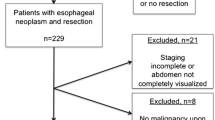
A total of 35 patients who received NAC followed by oesophagectomy, and underwent CT assessment pre- and post-NAC were included. Fat mass (FM), fat-free mass (FFM), subcutaneous fat to muscle ratio (FMR) and visceral to subcutaneous adipose tissue ratio (VA/SA) were derived from CT. Changes in FM, FFM, FMR, VA/SA and sarcopenia were correlated to chemotherapy dose reductions, postoperative complications, length of hospital stay (LOS), circumferential resection margin (CRM), pathological chemotherapy response, disease-free survival (DFS) and overall survival (OS).
Results
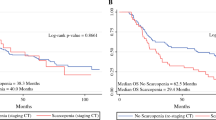
Nine (26 %) patients were sarcopenic before NAC and this increased to 15 (43 %) after NAC. Average weight loss was 3.7 % ± 6.4 (SD) in comparison to FM index (−1.2 ± 4.2), FFM index (−4.6 ± 6.8), FMR (−1.2 ± 24.3) and VA/SA (−62.3 ± 12.7). Changes in FM index (p = 0.022), FMR (p = 0.028), VA/SA (p = 0.024) and weight (p = 0.007) were significant univariable factors for CRM status. There was no significant association between changes in body composition and survival.
Conclusions
Loss of FM, differential loss of VA/SA and skeletal muscle were associated with risk of CRM positivity.
Key Points
• Changes in CT body composition occur after neoadjuvant chemotherapy in oesophageal cancer.
• Sarcopenia was more prevalent after neoadjuvant chemotherapy.
• Fat mass, fat-free mass and weight decreased after neoadjuvant chemotherapy.
• Changes in body composition were associated with CRM positivity.
• Changes in body composition did not affect perioperative complications and survival.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Cancer Research UK (2012) Oesophageal cancer survival statistics, Cancer Research UK, London. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/oesophagus/survival/. Accessed 2 Sept 2013
Berger B, Belka C (2009) Evidence-based radiation oncology: oesophagus. Radiother Oncol 92:276–290
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S (2006) Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr 96:895–901
Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P (2004) Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr 79:613–618
McClave SA, Snider HL, Spain DA (1999) Preoperative issues in clinical nutrition. Chest 115:64S–70S
Prado CM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13:3264–3268
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Prado CM, Lima IS, Baracos VE et al (2011) An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 67:93–101
Balentine CJ, Enriquez J, Fisher W et al (2010) Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg 14:1832–1837
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC (2009) Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15:6973–6979
Awad S, Tan BH, Cui H et al (2012) Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr 31:74–77
Bower MR, Martin RC 2nd (2009) Nutritional management during neoadjuvant therapy for esophageal cancer. J Surg Oncol 100:82–87
Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73:2680–2686
Mapstone NP (2007) Dataset for the histopathological reporting of oesophageal carcinoma, 2nd edn. Royal College of Pathologists, London
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122
Kvist H, Sjostrom L, Tylen U (1986) Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 10:53–67
Vehmas T, Kairemo KJ, Taavitsainen MJ (1996) Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord 20:570–573
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Ibrahim MM (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11:11–18
Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R (2006) Visceral fat is an independent predictor of all-cause mortality in men. Obesity 14:336–341
Moon HG, Ju YT, Jeong CY et al (2008) Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol 15:1918–1922
Clark W, Siegel EM, Chen YA et al (2013) Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 216:1070–1081
Sheetz KH, Zhao L, Holcombe SA et al (2013) Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 26:716–722
Acknowledgements
The scientific guarantor of this publication is Prof. Vicky Goh. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding by the Department of Health via the National Institute of Health Research Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust; and from the Comprehensive Cancer Imaging Centre, funded by the Cancer Research UK and Engineering and Physical Sciences Research Council in association with the Medical Research Council and Department of Health. Dr. Connie Yip receives funding support from the National Medical Research Council, Singapore. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was not required for this study because anonymised patient data was used with no intervention or patient contact. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Yip, C., Goh, V., Davies, A. et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 24, 998–1005 (2014). https://doi.org/10.1007/s00330-014-3110-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3110-4