Abstract
Purpose
To evaluate image quality using reduced contrast media (CM) volume in pre-TAVI assessment.
Methods
Forty-seven consecutive patients referred for pre-TAVI examination were evaluated. Patients were divided into two groups: group 1 BMI < 28 kg/m2 (n = 29); and group 2 BMI > 28 kg/m2 (n = 18). Patients received a combined scan protocol: retrospective ECG-gated helical CTA of the aortic root (80kVp) followed by a high-pitch spiral CTA (group 1: 70 kV; group 2: 80 kVp) from aortic arch to femoral arteries. All patients received one bolus of CM (300 mgI/ml): group 1: volume = 40 ml; flow rate = 3 ml/s, group 2: volume = 53 ml; flow rate = 4 ml/s. Attenuation values (HU) and contrast-to-noise ratio (CNR) were measured at the levels of the aortic root (helical) and peripheral arteries (high-pitch). Diagnostic image quality was considered sufficient at attenuation values > 250HU and CNR > 10.
Results
Diagnostic image quality for TAVI measurements was obtained in 46 patients. Mean attenuation values and CNR (HU ± SD) at the aortic root (helical) were: group 1: 381 ± 65HU and 13 ± 8; group 2: 442 ± 68HU and 10 ± 5. At the peripheral arteries (high-pitch), mean values were: group 1: 430 ± 117HU and 11 ± 6; group 2: 389 ± 102HU and 13 ± 6.
Conclusion
CM volume can be substantially reduced using low kVp protocols, while maintaining sufficient image quality for the evaluation of aortic root and peripheral access sites.
Key points
• Image quality could be maintained using low kVp scan protocols.
• Low kVp protocols reduce contrast media volume by 34–67 %.
• Less contrast media volume lowers the risk of contrast-induced nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcatheter aortic valve implantation (TAVI) is considered an effective treatment option for patients with severe and symptomatic valve stenosis not suitable for conventional valve replacement [1, 2]. Throughout the years, multi-detector row computed tomography (MDCT) has become the standard non-invasive imaging method in pre-TAVI assessment for aortic root dimensions and access site [3, 4]. To ensure technical success and optimal valve-prosthesis sizing, a dedicated scan technique including reliable contrast media (CM) injection protocols during pre-operative imaging are of utmost importance. However, the CM required may pose a risk for TAVI candidates who are frequently suffering from impaired renal function and considered to be at increased risk of contrast-induced nephropathy (CIN) [5, 6]. The European Society of Urogenital Radiology (ESUR) guidelines recommend expansion of the extracellular volume as one of the measures to prevent CIN [7]. However, aortic stenosis in TAVI candidates does not allow for fluid expansion due to the increased risk of subsequent heart failure.
Not only pre-existing renal insufficiency is correlated with increased risk of CIN, but also the use of increased CM volume [8] as well as the administration of multiple doses of intravascular CM within a short period of time (<24 hours) [9, 10].
TAVI protocols — as described in the literature — usually entail the injection of large CM volumes up to 120 ml to ensure optimal filling of the aortic root as well as the peripheral arteries [4, 11–13]. In the combination of ultra-fast data acquisition and use of low kVp protocols, this amount might be substantially reduced, offering patient-tailored CT protocols.
Thus, the aim of this study was to evaluate the use of low CM volumes in pre-TAVI CT examinations using low kVp settings in combination with iterative reconstruction techniques.
Materials and methods
Ethics
A waiver of written informed consent was obtained from the local ethical committee (METC, ref. 14-4-165).
Patient population
Between July 2014 and January 2015, 56 consecutive patients with severe symptomatic aortic stenosis were evaluated. All of them were referred from the cardiology outpatient department for pre-interventional assessment of aortic root dimensions and peripheral arteries. Patients with a body mass index (BMI) >35 kg/m2 received a divergent scan protocol and were, therefore, excluded (n = 9). The other 47 patients received a specific scan and injection protocol according to their BMI: group 1 BMI <28 kg/m2; (n = 29); group 2 BMI >28 kg/m2 (n = 18) [14, 15].
Other patient characteristics such as age, gender, height and weight were recorded.
MDCT scan protocol
All examinations were performed on a 2nd generation dual-source CT scanner (Definition Flash; Siemens Healthcare, Forchheim, Germany). Aortic root dimension assessment was carried out using a retrospective electrocardiogram (ECG)-gated helical scan in a caudo-cranial direction with parameters as follows: tube voltage 80 kVp; effective tube current 370 mAseff; rotation time 0.28 s; slice collimation 128 x 0.6 mm; pitch value 0.23.
Tube current was set to 370 mAseff, being the highest possible value for this scan protocol using this type of scanner. Image reconstruction of the entire cardiac cycle was done at the 20 % phase of the cardiac cycle with individually adapted field of view (FOV) at a 0.6-mm slice thickness, an increment of 0.4 mm using raw-data based iterative reconstruction [kernel I26f, SAFIRE (sinogram-affirmed iterative reconstruction), strength 3].
Directly afterwards, all patients received a non-ECG-triggered high-pitch spiral scan (flash) of the aorta in the cranio-caudal direction from the aortic arch to the femoral arteries (see Fig. 1). Scan parameters were as follows: reference tube-current-time product 400 mAsref; rotation time 0.28 s; slice collimation 128 × 0.6 mm; pitch value 3.0. Scan protocol varied for the two groups in tube voltage setting — group 1 at 70 kVp, group 2 at 80 kVp — and effective tube current was 90–122 mAseff (maximum possible values were 93 and 122 mAseff for 70 and 80 kVp, respectively). Images were reconstructed with individually adapted FOV at a 2-mm slice thickness with an increment of 1.4 mm using an I30f kernel (SAFIRE, strength 3; see Fig. 2). Dose modulation (CAREDose4D, Siemens) was used. In Table 1, all relevant scan parameters are summarized. The total scan time of the combined acquisition was 13–14 s: 6–7 s for retrospective ECG-gated acquisition, with a 5-s gap between acquisitions and 2 s for the high-pitch acquisition. In order to minimize the gap between acquisitions, the scan direction was adapted accordingly, starting with a caudo-cranial direction for the helical scan, followed by a cranio-caudal direction for the high-pitch spiral scan.
This figure shows the scout view with the planned anatomical range. The box with the dashed lines (1) indicates the retrospective ECG-gated acquisition of the heart in caudo-cranial direction. Box 2 indicates the high-pitch acquisition from the aortic arch to the femoral arteries in cranio-caudal direction
CM injection protocol
Monomeric, non-ionic, low-osmolar iodinated CM (300 mgI/ml; Iopromide; Bayer Healthcare, Berlin, Germany) was pre-warmed to a standardized 37 °C prior to injection in the antecubital vein using catheter sizes between 18–22 G (Sterican, Braun, Melsungen, Germany). Group 1 received a CM bolus of 40 ml followed by a saline flush of 36 ml, both at injection rate 3 ml/s. Group 2 received a CM bolus of 53 ml followed by a saline flush of 48 ml, both at injection rate 4 ml/s. Injection time of CM bolus and saline flush was kept constant in both groups (CM bolus: 13.3; saline flush: 12 s). Total iodine load (TIL, g) and iodine delivery rate (IDR, gI/s) were kept constant at 12 g and 0.9 gI/s for group 1; as well as 15.9 g and 1.2 gI/s for group 2. In order to determine time to peak (TTP) for accurate scan delay settings, the test-bolus technique was used with 10 ml of undiluted CM followed by 30 ml of saline, injected at 3 ml/s or 4 ml/s for group 1 and 2, respectively. Injection parameters such as injection pressure (psi), flow rate (ml/s) and total amount of CM (ml) were continuously monitored by a data acquisition program (Certegra™ Informatics Solution, Bayer) and read out after each injection.
Quantitative analysis
Radiation dose
The dose-length product (DLP) was recorded for each protocol to calculate the effective dose (mSv). The effective dose (E) was quantified by multiplying the DLP value and the combination of conversion coefficients (k) of the chest (k = 0.014 mSv/[mGy.cm]), abdominal (k = 0.015 mSv/[mGy.cm]) and pelvic (k = 0.017 mSv/[mGy.cm]) [16]. Because of the thoracic-abdominal-pelvic scan range, an average conversion factor (k = 0.015 mSv/[mGy.cm]) was used [16].
Image quality
Image analysis of both helical and flash scans was performed. For the helical scan, the 20 % time point of the cardiac cycle was used for image quality assessment [17]. Quality determination was based on the ability of images to provide diagnostically sufficient information of the aortic root (annulus and root diameters, distance from annulus to coronary ostia and length of valve leaflets) and aorto iliofemoral arteries for dimensional measurements (iliofemoral diameter). The latter is, as well as tortuosity, calcifications and/or atherosclerosis parts of the access assessment [18]. Image quality was determined from a combination of arterial enhancement, image noise and presence of artefacts.
Objective image quality measurements were performed by two experienced observers in consensus: attenuation in the region of interest (ROI), signal-to-noise ratio (SNR), and contrast to noise ratio (CNR). Circular ROIs (as large as possible) were placed at six levels of the aorta. The ascending aorta (AA) was evaluated from both the 80-kVp helical scan and the 70/80-kVp high-pitch spiral scan. The other five levels of the aorta [aortic arch (arch); descending aorta (DA); abdominal aorta (AAo); right and left common iliac arteries (RCIA and LCIA) and right and left common femoral arteries (RCFA and LCFA)], were evaluated from the 70/80-kVp high-pitch spiral scan only.
The SNR was defined as vessel enhancement in Hounsfield units (HU) divided by vessel enhancement standard deviation (SD). CNR was defined as vessel enhancement (HU) minus adjacent muscle tissue enhancement, divided by the adjacent muscle tissue enhancement SD. Diagnostic image quality was considered sufficient at attenuation values >200 HU [19, 20] and a CNR >3 [21].
Subjective image quality (IQ) of both the helical and the high-pitch acquisition was determined by rating the presence of artefacts using a four-point grading scale: 1 = non-diagnostic image quality; 2 = significantly reduced image quality due to major artefacts, but still diagnostic for assessment; 3 = good image quality with minor artefacts, and 4 = excellent image quality without artefacts.
Images were analysed using multiplanar reformation (MPR) with Syngo-Via™ software (Siemens).
Renal function
For the evaluation of renal function of this population, estimated glomerular filtration rate (eGFR; ml/min/1.73 m2) and serum creatinine (μmol/L) ≤12 months before the pre-TAVI CT scan were recorded according to the current hospital protocol.
Long-term renal function was evaluated by recording eGFR values ≥1–2 months after CTA.
TAVI procedure
For the evaluation of the placement of the valve prosthesis, the amount of patients treated by TAVI was recorded, as well as the survival rate after one month post-TAVI. In addition, the CT measurements from the radiological reports were compared to the actual size of the valve prosthesis. Reference-proposed guidelines show the best results when using an area-derived diameter or the mean diameter for valve sizing [4, 22]. For both balloon-expandable prosthesis and self-expandable prosthesis, over-sizing was recommended, by 10–15 %.
Statistical analysis
Data analysis was conducted using SPSS version 20.0 (SPSS Inc, Chicago, IL, USA). Continuous variables were reported as the mean ± standard deviation (SD), categorical values as proportions (%). The chi-square test was used to measure differences between categorical variables. Continuous variables were compared using an independent samples t-test and or analysis of variance (ANOVA) test. In addition, post hoc comparisons of differences between the vascular segments were performed. All reported p-values are two-sided, and a p-value of less than 0.05 is considered statistically significant.
Results
Baseline characteristics
Baseline characteristics are listed in Table 2. Group 1 consisted of 16 males and 13 females with average ages of 76 ± 9y and BMI 22 ± 3 kg/m2. Group 2 consisted of 5 males and 13 females with average age 75 ± 9y and BMI 31 ± 2 kg/m2.
Radiation dose and CM injection
Radiation dose and CM injection parameters are given in Table 3. Mean effective radiation dose for the combined scans was lower for group 1 (6 ± 2 mSv) as compared to group 2 (8 ± 2 mSv); p = 0.06. The effective dose for helical and high-pitch scans were respectively, 5 ± 1 mSv and 0.9 ± 0.1 mSv for group 1, and 6 ± 1 mSv and 1.8 ± 0.2 mSv for group 2. Mean volume, flow rate, peak flow rate, and peak pressure for groups 1 and group 2 were: 40.0 ± 0.1 and 53.0 ± 0.1 ml; 2.9 ± 0.0 and 3.9 ± 0.1 ml/s; 3.4 ± 0.0 and 4.2 ± 0.0 ml/s; 54 ± 8 and 68 ± 8, respectively.
Image quality
Of the image sets obtained from 47 patients, 46 were diagnostically sufficient for aortic root anatomy, cardiac structures, and aorto iliofemoral anatomy. In one patient, image quality was insufficient; this was due to streak artefacts caused by bilateral prosthesis material at the level of the hips and, therefore, at the level of the femoral arteries. Prosthesis material was present in one other patient (right hip) and femoral access could be properly depicted in this patient.
In helical scan images of the AA, sufficient enhancement levels (>200 HU) were reached for both groups. Significantly higher attenuation values were observed for group 2 (mean attenuation group 1: 381 ± 65 HU; group 2: 442 ± 68 HU; p = 0.004), whereas better SNR and CNR was observed for group 1 (SNR group 1 vs. group 2: 10 ± 5 vs 8 ± 2, p = 0.045; CNR group 1 vs. group 2: 13 ± 8, 10 ± 5, p = 0.152). High-pitch spiral scan images achieved sufficient overall enhancement levels (>200 HU) in both groups. A significantly higher mean attenuation was found at all levels of the aorta in group 1 (430 ± 117 HU) as compared to group 2 (389 ± 102 HU); p < 0.001. Post-hoc comparisons for each vascular segment revealed that the significant difference in attenuation existed at the levels of the RCIA, LCIA, RCFA and LCFA, with corresponding p-values 0.045, 0.016, 0.017 and 0.026. In none of the vascular segments was the mean attenuation <200 HU, nor were individual attenuation levels of <200 HU found at the level of the peripheral arteries (see Fig. 3). Significantly higher SNR and CNR values were found for group 2 (13 ± 6 and 13 ± 6) as compared to group 1 (11 ± 6 and 11 ± 6); p = 0.017 and p = 0.021, respectively (see Fig. 4). Post-hoc comparisons for each vascular segment revealed one mild significant difference between groups in the SNR at the level of the AAo, p = 0.048.
Box plots showing attenuation levels of each vascular segment. ‘’AA helical” (black) was measured in the helical cardiac scan. ‘’AA high-pitch” and the other levels (white) were measured in the high-pitch spiral scan of the aorta. * AA = ascending aorta; DA = descending aorta; AAo = abdominal aorta; RCIA and LCIA = right and left common femoral artery; RCFA and LCLA = right and left common femoral artery
Box plots show SNR (left) and CNR (right) levels of each vascular segment. ’AA helical” (black) was measured in the helical cardiac scan. ‘’AA high-pitch” and the other levels (white) were measured in the high-pitch spiral scan of the aorta. * AA = ascending aorta; DA = descending aorta; AAo = abdominal aorta; RCIA and LCIA = right and left common femoral artery; RCFA and LCLA = right and left common femoral artery
IQ scores of helical and high-pitch spiral acquisitions for both groups are listed in Table 4. No significant differences were found between IQ scores of both acquisitions, with p-values of 0.67 for group 1 and 0.37 for group 2. The IQ of each helical acquisition was diagnostic and IQ scores were significantly lower for group 2 compared to group 1 (p = 0.03). No significant difference in IQ was found between both groups for the high-pitch spiral scans (p = 0.07).
Renal function
The mean eGFR was 54 ± 8 ml/min/1.73 m2 and 55 ± 8 ml/min/1.73 m2 for group 1 and group 2, respectively. In 4 patients (9 %) the eGFR was <45 ml/min/1.73 m2. These values indicate the overall impaired renal function of this population. The mean eGFR ≥1–2 months after CTA was higher compared to the mean eGFR before CTA: 57 ± 4 ml/min/1.73 m2 and 57 ± 6 ml/min/1.73 m2 for group 1 and group 2, respectively. A constant eGFR was found in 32 patients, an increased eGFR in 13 patients and a decreased eGFR in two patients. In none of the cases was CIN detected after 1–2 months.
TAVI procedure
In total, 26 patients were treated by TAVI and the survival rate after one month was 100 %. Other patients underwent either an open procedure or conservative treatment. In all patients, the CT measurements were comparable to the size of the valve prosthesis implemented (Table 5).
Discussion
Vascular enhancement depends on three major factors — CM characteristics (e.g. flow rate), scanner related factors (e.g. tube voltage) and patient related factors (e.g. BMI). Especially, use of lower kVp is advantageous in terms of reducing CM, as the same amount of iodine will lead to a higher enhancement at a lower kVp [20], enabling substantial reduction in CM volume [23–25]. New scanner technologies facilitate lower kVp settings in a broader range of patients [26]. In addition, new reconstruction technologies, such as iterative reconstruction (IR), reduce image noise and, therefore, improve image quality [27–30] at lower kVp settings as well. Using BMI-adapted low-volume CM injection protocols in combination with low kVp scan settings provided diagnostically sufficient image quality in pre-TAVI assessment. Compared to the 80–120 ml of CM mostly used in TAVI protocols [13], we were able to reduce CM volume to 40 ml (=12 gI) and 53 ml (=16 gI) by using 70 kVp and 80 kVp settings, respectively. Further reduction (of 10 ml) would have been possible with use of bolus tracking, but a test bolus was chosen as additional information could be retrieved (e.g. cardiac output — beyond the scope of this paper) [31] and which might be used for further adaptation of the injection protocol [32].
Volume reduction up to 67 % still lead to sufficient attenuation values at all anatomical levels in all patients. In addition, SNR and CNR levels were found to be sufficient for both groups according to Leber et al. [21] who suggests CNR values >3 to be diagnostically acceptable.
Attenuation values, image noise and image quality ratios (SNR/CNR) are most affected by CM injection parameters, scan technique and patient body size [33]: Lowering IDR and TIL might compromise attenuation values and, therefore, image quality; Lowering radiation dose — by decreasing kVp or tube current settings — increases the amount of image noise; increased body size decreases attenuation values due to increased circulating blood volume and, simultaneously, image noise increases due to absorbing tissue. These factors necessitate optimized injection and scan parameters in terms of image quality. However, individual patient characteristics and indications for CTA need to be taken into account. For TAVI candidates, optimal image quality — meaning low image noise and high enhancement — is required at the level of the aortic root for aortic annulus evaluation. For the peripheral access route, higher noise levels are acceptable. In this respect, IQ scores were defined according to different criteria for each anatomic level in the current study. Comparisons between IQ at the level of the aortic root and the level of the peripheral arteries showed no significant differences in both groups. However, in one patient, image quality was found to be non-diagnostic at the level of the peripheral arteries due to the presence of prosthesis material. This could easily be picked up on the scout view and scan parameters can be adjusted accordingly (higher kVp settings). On the other hand, metallic artefacts do not necessarily hinder full evaluation of the vessels.
Optimizing radiation dose is of less importance in this particular patient population; stochastic effects can be regarded as negligible in patients with a mean age of 75/76 years. But the most challenging and important part of protocol optimization in a TAVI population with an inherent high number of impaired renal function is to reduce the risk of CIN by means of the most efficient use of CM [34, 35].
The use of reduced iodine load (IL) in pre-TAVI assessment has been investigated by several other groups. For example, Dubourg et al. [36] evaluated a combined CM injection protocol for an ECG-gated helical scan followed by a non-ECG-triggered high-pitch spiral scan. They used a dual energy acquisition with fast kVp switching (80–140 kVp) for the second acquisition, and were able to reduce the second bolus CM volume by 50 %. Total CM volume was reduced from 125 ml to 95 ml, which still results in a relatively high IL (33 gI using a CM concentration of 350 mgI/ml).
Wuest et al. [37] were able to reduce CM volume to 40 ml for CTA in TAVI candidates (14 gI with 350 mgI/ml). Patients with a BMI <30 received a 100-kVp scan protocol, with a BMI >30 a 120-kVp scan. Image quality (presence of motion artefacts, enhancement, image noise and CNR) for evaluation of aortic root complex was found to be diagnostic in 40 images from 42 patients.
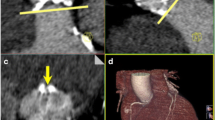
Azzalini et al. [38] tested the feasibility of an ultralow CM volume injection (20 ml = 7.4 gI with 370 mg/ml), in combination with a high-pitch scan mode in 8 patients. They found acceptable image quality using a 100- or 120-kVp setting (automated tube voltage setting according to patient body size). However, in these studies, only one scan — a high-pitch spiral acquisition — was performed for the evaluation of both aortic root and iliac dimensions, which compromises the dynamic assessment of the aortic valve and the annulus as retrospectively gated CTA — including a 20 % phase reconstruction — is recommended [17]. Figure 5 demonstrates the difference in dimensions of the annulus and valve between a 20 % phase reconstruction from a retrospective ECG-gated helical acquisition and a reconstruction from a high-pitch acquisition — as the latter provides no 20 % phase reconstruction. ECG-triggered high-pitch CTA is usually acquired during the diastolic phase, and should, therefore, be used exclusively for aorto iliofemoral CTA [2, 18, 36]. Although differences between phases of reconstruction might be small, they will lead to different measurements for the diameter and could, therefore, lead to different prosthesis sizing [17].
Images show the difference in the dimensions of the annulus and valve between reconstruction at the 20 % phase of the cardiac cycle using retrospective ECG-gated helical acquisition (upper) and reconstruction using the non ECG-gated high-pitch acquisition (bottom). The measurements for short and long diameter as well as perimeter were: 21 mm, 25 mm and 4.2 cm2, respectively for the helical acquisition and 19 mm, 24 mm and 3.8 cm2, respectively for the high-pitch acquisition
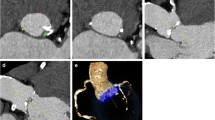
In none of the abovementioned studies was a combined scan protocol including a retrospective ECG-gated acquisition used. Either a high-pitch spiral scan acquisition was used in combination with low-volume injections, at the expense of a dynamic study; or a combined scan acquisition was used at the expense of CM volume reduction. One could argue that the use of only one scan acquisition is most advantageous as this could reduce scan time and, therefore, radiation dose and CM volume. However, an ECG-triggered, high-pitch scan acquisition is not desirable because of the reconstruction in the diastolic phase, as mentioned above. A complete retrospective ECG-gated acquisition is also not desirable as the scan time will greatly increase because of the decreased pitch factor compared to a high-pitch acquisition (0.23 vs. 3.0). As a result, radiation dose will increase and CM volume must also be adjusted according a prolonged injection time (see also Fig. 6).
This schematic figure shows the time to peak at the level of the ascending aorta as well as at the level of the peripheral arteries. The time in between was 12 s. Using the combined scan protocol, the fast second scan acquisition will again catch the bolus in the peripheral arteries. With only the retrospective ECG-gated acquisition scan time will be extended and the bolus will likely overtake the scan. A retrospective ECG-gated helical acquisition of the heart B gap between acquisitions C high-pitch acquisition of the aorta from aortic arch to femoral arteries
The combined scan protocol, as presented, will, therefore, ensure best image quality at the level of the aortic root for dedicated pre-interventional analysis in combination with a full picture of the access options for TAVI, while keeping radiation dose and TIL as low as possible.
In the current study, the overall renal function was not impaired after 1–2 months from CTA. This is in line with the study of McDonald et al., which stated that intravenous iodinated CM may not be the causative agent in impaired renal function after CM administration [39].
Limitations
One limitation is that serum creatinine data from 48–72 hours after CTA could not be retrieved in the majority of patients and, therefore, acute kidney failure could not be detected. However, the eGFR after 1–2 months showed no relevant changes in renal function compared to the eGFR before CTA. In addition, ultra-low kVp scan protocols as well as the ultra-fast acquisition mode are not available on every type of scanner and, in the current situation, the protocols are not applicable to every patient, due to technical limitations of the dedicated CT scanner used in this study: the maximum tube-current-time product for a high-pitch flash scan was 122 mAseff for 80 kVp and only 93 mAseff for 70 kVp. These low tube-current-time products present a problem because of resulting streak artefacts in the presence of prosthesis materials, or exceedingly high noise levels in obese patients.
Conclusion
Low kVp scan protocols allow for substantial reduction in CM volume as compared to common high-volume injection protocols for pre-TAVI CT examinations. Sufficient image quality was maintained for the evaluation of the aortic root and peripheral access site despite a CM volume reduction of 34–67 %. This may play an important role in terms of increased patient safety, as a lower iodine load is regarded as lowering the risk of CIN.
References
Webb JG, Pasupati S, Humphries K et al (2007) Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116:755–763
Holmes DR Jr, Mack MJ, Kaul S et al (2012) 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collabration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Thorac Cardiovasc Surg 144:e29–e84
Husser O, Holzamer A, Resch M et al (2013) Prosthesis sizing for transcatheter aortic valve implantation--comparison of three dimensional transesophageal echocardiography with multislice computed tomography. Int J Cardiol 168:3431–3438
Jilaihawi H, Kashif M, Fontana G et al (2012) Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 59:1275–1286
Yamamoto M, Hayashida K, Mouillet G et al (2013) Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv 6:479–486
Becker CR, Davidson C, Lameire N et al (2006) High-risk situations and procedures. Am J Cardiol 98:37K–41K
European Society of Urogenital Radiology web-site. ESUR Contrast Media Safety Committee. ESUR guidelines on contrast media, version 8.1. www.esur.org/guidelines/. Published 2012. Accessed May 2015
Mehran R, Nikolsky E (2006) Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. doi:10.1038/sj.ki.5000368:S11-15
Abujudeh HH, Gee MS, Kaewlai R (2009) In emergency situations, should serum creatinine be checked in all patients before performing second contrast CT examinations within 24 hours? J Am Coll Radiol 6:268–273
Trivedi H, Foley WD (2010) Contrast-induced nephropathy after a second contrast exposure. Ren Fail 32:796–801
Beeres M, Loch M, Schulz B et al (2013) Bolus timing in high-pitch CT angiography of the aorta. Eur J Radiol 82:1028–1033
Gurvitch R, Webb JG, Yuan R et al (2011) Aortic annulus diameter determination by multidetector computed tomography: reproducibility, applicability, and implications for transcatheter aortic valve implantation. JACC Cardiovasc Interv 4:1235–1245
Nguyen G, Leipsic J (2013) Cardiac computed tomography and computed tomography angiography in the evaluation of patients prior to transcatheter aortic valve implantation. Curr Opin Cardiol 28:497–504
Feuchtner GM, Jodocy D, Klauser A et al (2010) Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: a comparative study. Eur J Radiol 75:e51–e56
Winklehner A, Blume I, Winklhofer S et al (2013) Iterative reconstructions versus filtered back-projection for urinary stone detection in low-dose CT. Acad Radiol 20:1429–1435
Deak PD, Smal Y, Kalender WA (2010) Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 257:158–166
Jurencak T, Turek J, Kietselaer BL et al (2015) MDCT evaluation of aortic root and aortic valve prior to TAVI. What is the optimal imaging time point in the cardiac cycle? Eur Radiol. doi:10.1007/s00330-015-3607-5
Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA (2012) SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 6:366–380
Bae KT (2010) Optimization of contrast enhancement in thoracic MDCT. Radiol Clin N Am 48:9–29
Weininger M, Barraza JM, Kemper CA, Kalafut JF, Costello P, Schoepf UJ (2011) Cardiothoracic CT angiography: current contrast medium delivery strategies. AJR Am J Roentgenol 196:W260–W272
Leber AW, Knez A, Becker C et al (2003) Non-invasive intravenous coronary angiography using electron beam tomography and multislice computed tomography. Heart 89:633–639
Bloomfield GS, Gillam LD, Hahn RT et al (2012) A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc Imaging 5:441–455
Vlahos I, Chung R, Nair A, Morgan R (2012) Dual-energy CT: vascular applications. AJR Am J Roentgenol 199:S87–S97
Strocchi S, Vite C, Callegari L, Conte L (2006) Optimisation of multislice computed tomography protocols in angio-CT examinations. Radiol Med 111:238–244
Cao JX, Wang YM, Lu JG, Zhang Y, Wang P, Yang C (2014) Radiation and contrast agent doses reductions by using 80-kV tube voltage in coronary computed tomographic angiography: a comparative study. Eur J Radiol 83:309–314
Xia W, Wu JT, Yin XR, Wang ZJ, Wu HT (2014) CT angiography of the neck: value of contrast medium dose reduction with low tube voltage and high tube current in a 64-detector row CT. Clin Radiol 69:e183–e189
Pontana F, Pagniez J, Duhamel A et al (2013) Reduced-dose low-voltage chest CT angiography with Sinogram-affirmed iterative reconstruction versus standard-dose filtered back projection. Radiology 267:609–618
Wang R, Schoepf UJ, Wu R et al (2012) Image quality and radiation dose of low dose coronary CT angiography in obese patients: sinogram affirmed iterative reconstruction versus filtered back projection. Eur J Radiol 81:3141–3145
Korn A, Bender B, Fenchel M et al (2013) Sinogram affirmed iterative reconstruction in head CT: improvement of objective and subjective image quality with concomitant radiation dose reduction. Eur J Radiol 82:1431–1435
Baker ME, Dong F, Primak A et al (2012) Contrast-to-noise ratio and low-contrast object resolution on full- and low-dose MDCT: SAFIRE versus filtered back projection in a low-contrast object phantom and in the liver. AJR Am J Roentgenol 199:8–18
Mahnken AH, Klotz E, Hennemuth A et al (2003) Measurement of cardiac output from a test-bolus injection in multislice computed tomography. Eur Radiol 13:2498–2504
Mahnken AH, Rauscher A, Klotz E et al (2007) Quantitative prediction of contrast enhancement from test bolus data in cardiac MSCT. Eur Radiol 17:1310–1319
Bae KT (2010) Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 256:32–61
Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA (2009) Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol 192:711–718
Mack MJ, Brennan JM, Brindis R et al (2013) Outcomes following transcatheter aortic valve replacement in the United States. JAMA 310:2069–2077
Dubourg B, Caudron J, Lestrat JP et al (2014) Single-source dual-energy CT angiography with reduced iodine load in patients referred for aortoiliofemoral evaluation before transcatheter aortic valve implantation: impact on image quality and radiation dose. Eur Radiol 24:2659–2668
Wuest W, Anders K, Schuhbaeck A et al (2012) Dual source multidetector CT-angiography before Transcatheter Aortic Valve Implantation (TAVI) using a high-pitch spiral acquisition mode. Eur Radiol 22:51–58
Azzalini L, Abbara S, Ghoshhajra BB (2014) Ultra-low contrast computed tomographic angiography (CTA) with 20-mL total dose for transcatheter aortic valve implantation (TAVI) planning. J Comput Assist Tomogr 38:105–109
McDonald RJ, McDonald JS, Bida JP et al (2013) Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology 267:106–118
Acknowledgements
The scientific guarantor of this publication is Dr. M. Das.
Some authors (MD, JEW) of this manuscript declare institutional relationships with the following companies: Siemens AG, Bayer Healthcare, Philips, GE. The authors state that this work has not received any funding.
One of the authors has significant statistical expertise, but no complex statistical methods were necessary for this paper.
Institutional review board approval was obtained. Written informed consent was waived by the institutional review board.
Methodology: prospective observational study performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kok, M., Turek, J., Mihl, C. et al. Low contrast media volume in pre-TAVI CT examinations. Eur Radiol 26, 2426–2435 (2016). https://doi.org/10.1007/s00330-015-4080-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4080-x