Abstract
Objectives
Target therapy with BRAF/MEK inhibitors in metastatic melanoma is characterised by a high response rate; however, acquired resistance to treatment develops in many cases. We aimed to investigate if baseline total metabolic tumour volume (TMTV) and therapy-response assessment by [18F]FDG PET/CT have a prognostic role on progression-free survival (PFS) and overall survival (OS) in patients with metastatic melanoma receiving BRAF ± MEK inhibitors.
Methods
Fifty-seven patients who performed an [18F]FDG PET/CT at baseline and on treatment were retrospectively evaluated. A Cox proportional-hazard model was used to examine associations between OS and PFS with baseline clinical/PET parameters as well as for PET response.
Results
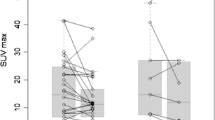
According to EORTC criteria, 34 patients were classified as responders (partial/complete metabolic response [PMR/CMR]) and 23 as non-responders (progressive/stable metabolic disease [PMD/SMD]). Baseline characteristics associated with a shorter PFS were more than two metastatic organ sites and TMTV > 56 cm3; the latter was the only independent feature at multivariate analysis. Patients achieving a CMR were associated with a prolonged PFS compared with those with PMR (median PFS 42.9 vs 8.8 months; p = 0.009). Disease progression occurred in new-onset disease sites in 87.5% of CMR, 7.1% of PMR and 34.8% of PMD/SMD (p < 0.001). High baseline TMTV and lack of treatment response were independent prognostic factors for OS, stratifying patients in three different prognostic classes (median OS 6.7, 18.3 and 102.2 months, respectively).
Conclusions
Baseline TMTV and metabolic response may be useful prognostic indicators for PFS and OS in patients with advanced melanoma treated with BRAF/MEK inhibitors.
Key Points
• In a retrospective cohort of 57 metastatic melanoma patients treated with BRAF/MEK inhibitors, a TMTV > 56 cm 3 at baseline [ 18 F]FDG PET/CT was significantly correlated with a shorter PFS and OS.
• The combined use of baseline TMTV along with PET response during treatment allowed for the identification of three groups of patients with very different median OS.




Similar content being viewed by others
Change history
09 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00330-022-08672-z
Abbreviations
- [18F]FDG PET/CT:
-
2-[18F]fluoro-2-deoxy-d-glucose positron emission/computed tomography
- BRAFi:
-
BRAF inhibitors
- CMR:
-
Complete metabolic response
- MEKi:
-
MEK inhibitors
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PMD:
-
Progressive metabolic disease
- PMR:
-
Partial metabolic response
- SMD:
-
Stable metabolic disease
- TMTV:
-
Total metabolic tumour volume
References
Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U, ESMO Guidelines Committee (2019) Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1884–1901. https://doi.org/10.1093/annonc/mdz411
Barth A, Wanek LA, Morton DL (1995) Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg 181:193–201
Ugurel S, Röhmel J, Ascierto PA et al (2020) Survival of patients with advanced metastatic melanoma: the impact of MAP kinase pathway inhibition and immune checkpoint inhibition - update 2019. Eur J Cancer 130:126–138. https://doi.org/10.1016/j.ejca.2020.02.021
Robert C, Grob JJ, Stroyakovskiy D et al (2019) Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626–636. https://doi.org/10.1056/NEJMoa1904059
Bisschop C, de Heer EC, Brouwers AH, Hospers GAP, Jalving M (2020) Rational use of 18F-FDG PET/CT in patients with advanced cutaneous melanoma: a systematic review. Crit Rev Oncol Hematol 153:103044. https://doi.org/10.1016/j.critrevonc.2020.103044
Theodosakis N, Held MA, Marzuka-Alcala A et al (2015) BRAF Inhibition decreases cellular glucose uptake in melanoma in association with reduction in cell volume. Mol Cancer Ther 14:1680–92. https://doi.org/10.1158/1535-7163.MCT-15-0080
McArthur GA, Puzanov I, Amaravadi R et al (2012) Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J Clin Oncol 30:1628–34. https://doi.org/10.1200/JCO.2011.39.1938
Carlino MS, Saunders CA, Haydu LE et al (2013) (18)F-labelled fluorodeoxyglucose-positron emission tomography (FDG-PET) heterogeneity of response is prognostic in dabrafenib treated BRAF mutant metastatic melanoma. Eur J Cancer 49:395–402. https://doi.org/10.1016/j.ejca.2012.08.018
Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–82. https://doi.org/10.1016/s0959-8049(99)00229-4
Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, Pellot-Barakat C, Soussan M, Frouin F, Buvat I (2018) LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Research 78(16):4786–4789
Trunzer K, Pavlick AC, Schuchter L et al (2013) Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol 31:1767–74. https://doi.org/10.1200/JCO.2012.44.7888
Rizos H, Menzies AM, Pupo GM et al (2014) BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res 20:1965–77. https://doi.org/10.1158/1078-0432.CCR-13-3122
Long GV, Flaherty KT, Stroyakovskiy D et al (2017) Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631–1639. https://doi.org/10.1093/annonc/mdx176
Schadendorf D, Long GV, Stroiakovski D et al (2017) Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 82:45–55. https://doi.org/10.1016/j.ejca.2017.05.033
Parmenter TJ, Kleinschmidt M, Kinross KM et al (2014) Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov 4:423–33. https://doi.org/10.1158/2159-8290.CD-13-0440
Annovazzi A, Vari S, Giannarelli D et al (2020) Comparison of 18F-FDG PET/CT criteria for the prediction of therapy response and clinical outcome in patients with metastatic melanoma treated with ipilimumab and PD-1 inhibitors. Clin Nucl Med 45:187–194. https://doi.org/10.1097/RLU.0000000000002921
Long GV, Weber JS, Infante JR et al (2016) Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 34:871–878. https://doi.org/10.1200/JCO.2015.62.9345
Chen G, Chakravarti N, Aardalen K et al (2014) Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res 20:5537–5546. https://doi.org/10.1158/1078-0432.CCR-13-3003
Amaral T, Sinnberg T, Meier F et al (2017) The mitogen-activated protein kinase pathway in melanoma part I - activation and primary resistance mechanisms to BRAF inhibition. Eur J Cancer 73:85–92. https://doi.org/10.1016/j.ejca.2016.12.010
Gutzmer R, Vordermark D, Hassel JC et al (2020) Melanoma brain metastases - interdisciplinary management recommendations 2020. Cancer Treat Rev 89:102083. https://doi.org/10.1016/j.ctrv.2020.102083
Woff E, Hendlisz A, Ameye L et al (2019) Validation of metabolically active tumor volume and total lesion glycolysis as 18F-FDG PET/CT–derived prognostic biomarkers in chemorefractory metastatic colorectal cancer. J Nucl Med 60:178–184. https://doi.org/10.2967/jnumed.118.210161
Lim R, Eaton A, Lee NY et al (2012) 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med 53:1506–13. https://doi.org/10.2967/jnumed.111.101402
Van de Wiele C, Kruse V, Smeets P, Sathekge M, Maes A (2013) Predictive and prognostic value of metabolic tumour volume and total lesion glycolysis in solid tumours. Eur J Nucl Med Mol Imaging 40:290–301. https://doi.org/10.1007/s00259-012-2280-z
Kwee SA, Lim J, Watanabe A, Kromer-Baker K, Coel MN (2014) Prognosis related to metastatic burden measured by 18F-fluorocholine PET/CT in castration-resistant prostate cancer. J Nucl Med 55:905–10. https://doi.org/10.2967/jnumed.113.135194
Ito K, Schöder H, Teng R et al (2019) Prognostic value of baseline metabolic tumor volume measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging 46:930–939. https://doi.org/10.1007/s00259-018-4211-0
Gershenwald JE, Scolyer RA, Hess KR, et al (2017) Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472–492. https://doi.org/10.3322/caac.21409
Keilholz U, Ascierto PA, Dummer R et al (2020) ESMO consensus conference recommendations on the management of metastatic melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol 31:1435–1448. https://doi.org/10.1016/j.annonc.2020.07.004
Ascierto PA, Ferrucci PF, Fisher R et al (2019) Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 25:941–946. https://doi.org/10.1038/s41591-019-0448-9
Ribas A, Lawrence D, Atkinson V et al (2019) Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med 25:936–940. https://doi.org/10.1038/s41591-019-0476-5
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Alessio Annovazzi.
Conflict of Interest
Dr. Virginia Ferraresi: consulting or advisory role—Bristol Myers Squibb, Novartis, MSD; speaker—Bristol Myers Squibb, Novartis, Pierre Fabre; travel/accommodations: Bristol Myers Squibb, Pierre Fabre, MSD. Dott. Michelangelo Russilo: speaker—Novartis, Pierre Fabre. All remaining authors have declared no conflicts of interest.
Statistics and Biometry
Two of the authors have significant statistical expertise.
Informed Consent
Given the retrospective design of the study, the ethics committee allowed the use and processing of the patients’ clinical data even in the absence of written informed consent.
Ethical Approval
The study was approved by the local institutional ethics committee (prot. n° 1460/21) and it was performed in accordance with ethical standards.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The reference 'C Nioche, F Orlhac, S Boughdad, S Reuzé, J Goya-Outi, C Robert, C Pellot-Barakat, M Soussan, F Frouin, and I Buvat. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Research 2018; 78(16):4786-4789' was added.
Rights and permissions
About this article
Cite this article
Annovazzi, A., Ferraresi, V., Rea, S. et al. Prognostic value of total metabolic tumour volume and therapy-response assessment by [18F]FDG PET/CT in patients with metastatic melanoma treated with BRAF/MEK inhibitors. Eur Radiol 32, 3398–3407 (2022). https://doi.org/10.1007/s00330-021-08355-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08355-1