Abstract
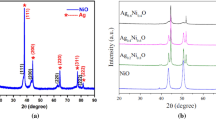
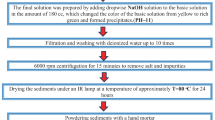
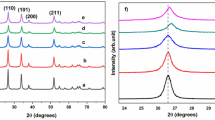
Many studies have concentrated on exploring behaviors of nickel silver oxide nanoparticles using various routes of fabrication. Thermal treatment technique has never been utilized to fabricate nickel oxide silver oxide nanoparticles. In this research, binary (NiO)0.4 (Ag2O)0.6 nanoparticles were synthesized using the thermal treatment method due to its attractive advantages such as low cost, eco-friendly, and purity of nanoparticles. The structural, morphological, and optical behaviors of these nanoparticles were investigated at different calcined temperatures. X-ray diffraction (XRD), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX), X-ray photoelectron spectroscopy (XPS), ultraviolet–visible spectroscopy (UV–Vis), and photoluminescence (PL) were the techniques used to characterize the synthesized nanoparticles. XRD was conducted at different calcined temperatures. The crystallite size was increased from 25.4 nm to 37.0 nm as the calcined temperature increased from 500 °C to 800 °C. Also, TEM results verified that the mean particle size was enlarged as the calcined temperatures increased. Two band gaps were found for each temperature, which were decreased from (3.05, 2.45) to (2.70, 1.95) eV as the temperature varied from 500 to 800 °C, respectively. Broadbands were observed by PL spectra, and the intensity of two emission peaks was also increased at higher temperatures. The results approved the successful formation of binary (NiO)0.4 (Ag2O)0.6 nanoparticles by a novel facile synthesis route. These nanoparticles are likely to have various applications, especially optical applications due to the formation of two band gaps.








Similar content being viewed by others
References
M. Tadic, D. Nikolic, M. Panjan, G.R. Blake, Magnetic properties of NiO (nickel oxide) nanoparticles: Blocking temperature and Neel temperature. J. Alloys Compd. 647, 1061–1068 (2015). https://doi.org/10.1016/j.jallcom.2015.06.027
G.T. Anand, R. Nithiyavathi, R. Ramesh, S. John Sundaram, K. Kaviyarasu, Structural and optical properties of nickel oxide nanoparticles: Investigation of antimicrobial applications, Surfaces and Interfaces. 18 (2020) 100460. https://doi.org/10.1016/j.surfin.2020.100460.
V.E. Gurenko, V.I. Popkov, A.A. Lobinsky, Synthesis of NiO granular nanospheres as a novel material for high-performance supercapacitors. Mater. Lett. 279, 128478 (2020). https://doi.org/10.1016/j.matlet.2020.128478
A.D. Khalaji, M. Jarosova, P. Machek, K. Chen, D. Xue, Facile synthesis, characterization and electrochemical performance of nickel oxide nanoparticles prepared by thermal decomposition. Scr. Mater. 181, 53–57 (2020). https://doi.org/10.1016/j.scriptamat.2020.02.015
J. Singh, S. Lee, S. Kim, S.P. Singh, J. Kim, A.K. Rai, Fabrication of 1D mesoporous NiO nano-rods as high capacity and long-life anode material for lithium ion batteries. J. Alloys Compd. 850, 156755 (2021). https://doi.org/10.1016/j.jallcom.2020.156755
A.M. Abdallah, H. Basma, R. Awad, Preparation, Characterization, and Application of Nickel Oxide Nanoparticles in Glucose and Lactose Biosensors. Mod. Appl. Sci. 13, 99 (2019). https://doi.org/10.5539/mas.v13n6p99
K. Deevi, V.S.R. Immareddy, Synthesis and characterization of optically transparent nickel oxide nanoparticles as a hole transport material for hybrid perovskite solar cells. J. Mater. Sci. Mater. Electron. 30, 6242–6248 (2019). https://doi.org/10.1007/s10854-019-00927-8
J.S. Fain, J.W. Mares, S.M. Weiss, Size-controlled nickel oxide nanoparticle synthesis using mesoporous silicon thin films, J. Nanoparticle Res. 17 (2015). https://doi.org/10.1007/s11051-015-3122-2.
K. Kaviyarasu, E. Manikandan, J. Kennedy, M. Jayachandran, R. Ladchumananandasiivam, U.U. De Gomes, M. Maaza, Synthesis and characterization studies of NiO nanorods for enhancing solar cell efficiency using photon upconversion materials. Ceram. Int. 42, 8385–8394 (2016). https://doi.org/10.1016/j.ceramint.2016.02.054
J. Fowsiya, G. Madhumitha, Biomolecules derived from Carissa edulis for the microwave assisted synthesis of Ag2O nanoparticles: a study against S. incertulas, C. medinalis and S. mauritia, J. Clust. Sci. 30 (2019) 1243–1252. https://doi.org/10.1007/s10876-019-01627-3.
S. Haq, K.A. Yasin, W. Rehman, M. Waseem, M.N. Ahmed, M.I. Shahzad, N. Shahzad, A. Shah, M.U. Rehman, B. Khan, Green synthesis of silver oxide nanostructures and investigation of their synergistic effect with Moxifloxacin against selected microorganisms. J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-020-01763-8
R. Li, Z. Chen, N. Ren, Y. Wang, Y. Wang, F. Yu, Biosynthesis of silver oxide nanoparticles and their photocatalytic and antimicrobial activity evaluation for wound healing applications in nursing care. J. Photochem. Photobiol. B Biol. 199, 111593 (2019). https://doi.org/10.1016/j.jphotobiol.2019.111593
V. Manikandan, P. Velmurugan, J.P.W. Chang, Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech. 7 (2017) 1–9. https://doi.org/10.1007/s13205-017-0670-4.
B.N. Rashmi, S.F. Harlapur, B. Avinash, C.R. Ravikumar, H.P. Nagaswarupa, M.R. Anil Kumar, K. Gurushantha, M.S. Santosh, Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic and biological studies. Inorg. Chem. Commun. 111 (2020) 107580. https://doi.org/10.1016/j.inoche.2019.107580.
S.P. Vinay, G. Udayabhanu, C.P. Nagaraju, N. Chandrappa, Chandrasekhar, Novel Gomutra (cow urine) mediated synthesis of silver oxide nanoparticles and their enhanced photocatalytic, photoluminescence and antibacterial studies. J. Sci. Adv. Mater. Devices. 4, 392–399 (2019). https://doi.org/10.1016/j.jsamd.2019.08.004
G. Pradheesh, S. Suresh, J. Suresh, V. Alexramani, Antimicrobial and Anticancer Activity Studies on Green Synthesized Silver Oxide Nanoparticles from the Medicinal Plant Cyathea nilgiriensis Holttum. Int. J. Pharm. Investig. 10, 146–150 (2020). https://doi.org/10.5530/ijpi.2020.2.27
S. Iqbal, M. Fakhar-e-alam, F. Akbar, M. Sha, M. Atif, N. Amin, M. Ismail, A. Hanif, W.A. Farooq, Application of silver oxide nanoparticles for the treatment of cancer 1189, 203–209 (2019). https://doi.org/10.1016/j.molstruc.2019.04.041
G. Maheshwaran, A. Nivedhitha Bharathi, M. Malai Selvi, M. Krishna Kumar, R. Mohan Kumar, S. Sudhahar, Green synthesis of Silver oxide nanoparticles using Zephyranthes Rosea flower extract and evaluation of biological activities. J. Environ. Chem. Eng. 8 (2020) 104137. https://doi.org/10.1016/j.jece.2020.104137.
T. Zahra, K.S. Ahmad, Structural, optical and electrochemical studies of organo-templated wet synthesis of cubic shaped nickel oxide nanoparticles. Optik (Stuttg). 205, 164241 (2020). https://doi.org/10.1016/j.ijleo.2020.164241
A.A. Olajire, A.A. Mohammed, Green synthesis of nickel oxide nanoparticles and studies of their photocatalytic activity in degradation of polyethylene films. Adv. Powder Technol. 31, 211–218 (2020). https://doi.org/10.1016/j.apt.2019.10.012
N.M. Al-Hada, H.M. Kamari, M.A. Saleh, M.H. Flaifel, A.M. Al-Ghaili, H. Kasim, A.A. Baqer, E. Saion, W. Jihua, Morphological, structural and optical behaviour of PVA capped binary (NiO)0.5 (Cr2O3)0.5 nanoparticles produced via single step based thermal technique, Results Phys. 17 (2020) 103059. https://doi.org/10.1016/j.rinp.2020.103059.
D. Thakur, A. Sharma, D.S. Rana, N. Thakur, D. Singh, T. Tamulevicius, M. Andrulevicius, Facile synthesis of silver-doped zinc oxide nanostructures as efficient scaffolds for detection of p-Nitrophenol. (2020).
K. Prikhodko, A. Nasriddinov, S. Vladimirova, M. Rumyantseva, A. Gaskov, Nanocrystalline oxides Ni x Co3− x O4 : Sub-ppm H 2 S Sensing and Humidity Effect. (2021).
Z. Fereshteh, M. Salavati-Niasari, K. Saberyan, S.M. Hosseinpour-Mashkani, F. Tavakoli, Synthesis of nickel oxide nanoparticles from thermal decomposition of a new precursor. J. Clust. Sci. 23, 577–583 (2012). https://doi.org/10.1007/s10876-012-0477-8
M. Hashem, E. Saion, N.M. Al-Hada, H.M. Kamari, A.H. Shaari, Z.A. Talib, S.B. Paiman, M.A. Kamarudeen, Fabrication and characterization of semiconductor nickel oxide (NiO) nanoparticles manufactured using a facile thermal treatment. Results Phys. 6, 1024–1030 (2016). https://doi.org/10.1016/j.rinp.2016.11.031
Z.H. Dhoondia, H. Chakraborty, Lactobacillus mediated synthesis of silver oxide nanoparticles, Nanomater. Nanotechnol. 2 (2012). https://doi.org/10.5772/55741.
M.R.H. Siddiqui, S.F. Adil, M.E. Assal, R. Ali, A. Al-Warthan, Synthesis and characterization of silver oxide and silver chloride nanoparticles with high thermal stability, Asian J. Chem. 25 (2013) 3405–3409. https://doi.org/10.14233/ajchem.2013.13874.
M.M. Rahman, S.B. Khan, A. Jamal, M. Faisal, A.M. Asiri, Highly sensitive methanol chemical sensor based on undoped silver oxide nanoparticles prepared by a solution method, (2012) 99–106. https://doi.org/10.1007/s00604-012-0817-2.
H. Baksh, J.A. Buledi, N.H. Khand, A.R. Solangi, A. Mallah, S.T. Sherazi, M.I. Abro, Ultra-selective determination of carbofuran by electrochemical sensor based on nickel oxide nanoparticles stabilized by ionic liquid. Monatshefte Fur Chemie. 151, 1689–1696 (2020). https://doi.org/10.1007/s00706-020-02704-4
L.M. Lyu, W.C. Wang, M.H. Huang, Synthesis of Ag2O nanocrystals with systematic shape evolution from cubic to hexapod structures and their surface properties. Chem. A Eur. J. 16, 14167–14174 (2010). https://doi.org/10.1002/chem.201000563
W.M. Shume, H.C.A. Murthy, E.A. Zereffa, A review on synthesis and characterization of Ag2O nanoparticles for photocatalytic applications. J. Chem. 2020 (2020). https://doi.org/10.1155/2020/5039479.
S. Ahmad, H. Rashid, Q. Jalil, S. Munir, S. Khan, Barkatullah, R. Ullah, A.A. Shahat, A.A.N.A. A-Mishari, A.A. Shahat, H.M. Mahmood, A. Bari, Polymers encapsulated aspirin loaded silver oxide nanoparticles: Synthesis, characterization and its bio-applications, Sains Malaysiana. 48 (2019) 1887–1897. https://doi.org/10.17576/jsm-2019-4809-09.
S.M. Hosseinpour-Mashkani, M. Ramezani, Silver and silver oxide nanoparticles: synthesis and characterization by thermal decomposition. Mater. Lett. 130, 259–262 (2014). https://doi.org/10.1016/j.matlet.2014.05.133
N.L. Yong, A. Ahmad, A.W. Mohammad, Synthesis and characterization of silver oxide nanoparticles by a novel method 4, 155–158 (2013)
F.T. Thema, E. Manikandan, A. Gurib-Fakim, M. Maaza, Single phase Bunsenite NiO nanoparticles green synthesis by Agathosma betulina natural extract. J. Alloys Compd. 657, 655–661 (2016). https://doi.org/10.1016/j.jallcom.2015.09.227
M.I. Din, A.G. Nabi, A. Rani, A. Aihetasham, M. Mukhtar, Single step green synthesis of stable nickel and nickel oxide nanoparticles from Calotropis gigantea: Catalytic and antimicrobial potentials. Environ. Nanotechnol. Monit. Manag. 9, 29–36 (2018). https://doi.org/10.1016/j.enmm.2017.11.005
B. Yu, T. Ayvalı, E. Raine, T. Li, M.M.J. Li, J. Zheng, S. Wu, A.A. Bagabas, S.C.E. Tsang, Enhanced propylene oxide selectivity for gas phase direct propylene epoxidation by lattice expansion of silver atoms on nickel nanoparticles. Appl. Catal. B Environ. 243, 304–312 (2019). https://doi.org/10.1016/j.apcatb.2018.10.061
M.M. Mohammadi, S.S. Gunturi, S. Shao, S. Konda, R.D. Buchner, M.T. Swihart, Flame-synthesized nickel-silver nanoparticle inks provide high conductivity without sintering. Chem. Eng. J. 372, 648–655 (2019). https://doi.org/10.1016/j.cej.2019.04.141
J.J. Jing, J. Xie, G.Y. Chen, W.H. Li, M.M. Zhang, Preparation of nickel–silver core–shell nanoparticles by liquid-phase reduction for use in conductive paste. J. Exp. Nanosci. 10, 1347–1356 (2015). https://doi.org/10.1080/17458080.2015.1012751
S. Senapati, S.K. Srivastava, S.B. Singh, H.N. Mishra, Magnetic Ni/Ag core-shell nanostructure from prickly Ni nanowire precursor and its catalytic and antibacterial activity. J. Mater. Chem. 22, 6899–6906 (2012). https://doi.org/10.1039/c2jm00143h
Y. Thaver, S.O. Oseni, G. Tessema, Silver doped nickel oxide nanocomposite and photon harvesting enhancement in bulkheterojunction organic solar cell. Sol. Energy. 214, 11–18 (2021). https://doi.org/10.1016/j.solener.2020.11.044
S. Ghazal, A. Akbari, H.A. Hosseini, Z. Sabouri, F. Forouzanfar, M. Khatami, M. Darroudi, Biosynthesis of silver-doped nickel oxide nanoparticles and evaluation of their photocatalytic and cytotoxicity properties. Appl. Phys. A Mater. Sci. Process. 126, 1–8 (2020). https://doi.org/10.1007/s00339-020-03664-6
C. Liu, D. Xie, P. Liu, S. Xie, S. Wang, F. Cheng, M. Zhang, L. Wang, Voltammetric determination of levofloxacin using silver nanoparticles deposited on a thin nickel oxide porous film. Microchim. Acta. 186 (2019). https://doi.org/10.1007/s00604-018-3146-2.
S. Nagamuthu, K.S. Ryu, Synthesis of Ag/NiO Honeycomb structured nanoarrays as the electrode material for high performance asymmetric supercapacitor devices. Sci. Rep. 9, 1–11 (2019). https://doi.org/10.1038/s41598-019-41446-0
M.Z. Iqbal, R.J. Kriek, Silver/Nickel Oxide (Ag/NiO) Nanocomposites produced via a Citrate Sol-Gel route as electrocatalyst for the oxygen evolution reaction (OER) in alkaline medium. Electrocatalysis 9, 279–286 (2018). https://doi.org/10.1007/s12678-018-0455-5
W. Zhao, N. Du, H. Zhang, D. Yang, Silver-nickel oxide core-shell nanoflower arrays as high-performance anode for lithium-ion batteries. J. Power Sources. 285, 131–136 (2015). https://doi.org/10.1016/j.jpowsour.2015.03.088
W. Zhao, N. Du, H. Zhang, D. Yang, Silver-nickel oxide core-shell nanoparticle array electrode with enhanced lithium-storage performance. Electrochim. Acta. 174, 893–899 (2015). https://doi.org/10.1016/j.electacta.2015.04.156
N.M. Al-Hada, E. Saion, H.M. Kamari, M.H. Flaifel, A.H. Shaari, Z.A. Talib, N. Abdullahi, A.A. Baqer, A. Kharazmi, Structural, morphological and optical behaviour of PVP capped binary (ZnO)0.4 (CdO)0.6 nanoparticles synthesised by a facile thermal route. Mater. Sci. Semicond. Process. 53 (2016) 56–65. https://doi.org/10.1016/j.mssp.2016.06.004.
R. Bharthasaradhi, L.C. Nehru, Structural and phase transition of α- Al2O3 powders obtained by co-precipitation method. Phase Transitions 89, 77–83 (2016). https://doi.org/10.1080/01411594.2015.1072628
M.A. Abd, A.H. Ali, A.N. Abd, Investigation and characterization of simple chemical method synthesized CdO-NiO nancomposite. J. Phys. Conf. Ser. 1234 (2019). https://doi.org/10.1088/1742-6596/1234/1/012051.
D.A. Svintsitskiy, M.K. Lazarev, T.Y. Kardash, E.A. Fedorova, E.M. Slavinskaya, A.I. Boronin, Mixed silver-nickel oxide AgNiO2: probing by CO during XPS study. J. Chem. Phys. 152 (2020). https://doi.org/10.1063/1.5138237.
A.Y. Vasil’kov, R.I. Dovnar, S.M. Smotryn, N.N. Iaskevich, A. V. Naumkin, Plasmon resonance of silver nanoparticles as a method of increasing their antibacterial action. Antibiotics. 7 (2018). https://doi.org/10.3390/antibiotics7030080.
N.M. Al-Hada, A.M. Al-Ghaili, H. Kasim, M.A. Saleh, H. Baqiah, J. Liu, J. Wang, Nanofabrication of (Cr2O3)x (NiO)1–x and the impact of precursor concentrations on nanoparticles conduct. J. Mater. Res. Technol. 11, 252–263 (2021). https://doi.org/10.1016/j.jmrt.2021.01.007
N.M. Al-Hada, H.M. Kamari, M.A. Saleh, M.H. Flaifel, A.M. Al-Ghaili, H. Kasim, A.A. Baqer, E. Saion, W. Jihua, Morphological, structural and optical behaviour of PVA capped binary (NiO)0.5 (Cr2O3)0.5 nanoparticles produced via single step based thermal technique. Results Phys. 17 (2020) 103059. https://doi.org/10.1016/j.rinp.2020.103059.
A.A. Baqer, K.A. Matori, N.M. Al-Hada, A.H. Shaari, H.M. Kamari, E. Saion, J.L.Y. Chyi, C.A.C. Abdullah, Synthesis and characterization of binary (CuO)0.6(CeO2)0.4 nanoparticles via a simple heat treatment method. Results Phys. 9 (2018) 471–478. https://doi.org/10.1016/j.rinp.2018.02.079.
A. Pahlavan, H. Karimi-Maleh, F. Karimi, M.A. Amiri, Z. Khoshnama, M.R. Shahmiri, M. Keyvanfard, Application of CdO nanoparticle ionic liquid modified carbon paste electrode as a high sensitive biosensor for square wave voltammetric determination of NADH. Mater. Sci. Eng. C. 45, 210–215 (2014). https://doi.org/10.1016/j.msec.2014.09.013
S. Muhamad, H. Mohamed Kamari, N.M. Al-Hada, C.A.C. Abdullah, N.N.S. Nidzam, Fabrication of binary (ZnO)x(TiO2)1−x nanoparticles via thermal treatment route and evaluating the impact of various molar concentrations on the structure and optical behaviors. Appl. Phys. A Mater. Sci. Process. 126 (2020) 1–15. https://doi.org/10.1007/s00339-020-03701-4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Absi, E., Saleh, M.A., Al-Hada, N.M. et al. Binary nickel and silver oxides by thermal route: preparation and characterization. Appl. Phys. A 127, 606 (2021). https://doi.org/10.1007/s00339-021-04775-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04775-4