Abstract
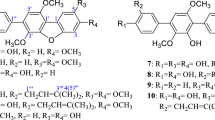
Bioassay-guided fractionation of the crude extract from Penicillium commune SD-118, a fungus obtained from a deep-sea sediment sample, resulted in the isolation of a known antibacterial compound, xanthocillin X (1), and 14 other known compounds comprising three steroids (2–4), two ceramides (5 and 6), six aromatic compounds (7–12), and three alkaloids (13–15). Xanthocillin X (1) was isolated for the first time from a marine fungus. In the bioassay, xanthocillin X (1) displayed remarkable antimicrobial activity against Staphylococcus aureus and Escherichia coli, and significant cytotoxicity against MCF-7, HepG2, H460, Hela, Du145, and MDA-MB-231 cell lines. Meleagrin (15) exhibited cytotoxicity against HepG2, Hela, Du145, and MDA-MB-231 cell lines. This is the first report of the cytotoxicity of xanthocillin X (1).
Similar content being viewed by others
References
Adler J H, Young M, Nes W R. 1977. Determination of the absolute configuration at C-20 and C-24 of ergosterol in ascomycetes and basidiomycetes by proton magnetic resonance spectroscopy. Lipids, 12(4): 364–366.
Al-Burtamani S K S, Fatope M O, Marwah R G, Onifade A K, Al-Saidi S H. 2005. Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J. Ethnopharmacol., 96(1–2): 107–112.
Amir-Heidari B, Micklefield J. 2007. NMR confirmation that tryptophan dehydrogenation occurs with Syn stereochemistry during the biosynthesis of CDA in Streptomyces coelicolor. J. Org. Chem., 72(23): 8 950–8 953.
Andersen R J, Wolfe M S, Faulkner D J. 1974. Autotoxic antibiotic production by a marine Chromobacterium. Marine Biology, 27(4): 281–285.
Bergeron R J, Cavanaugh P F Jr, Kline S J, Hughes R G Jr, Elliott G T, Porter C W. 1984. Antineoplastic and antiherpetic activity of spermidine catecholamide iron chelators. Biochem. Biophys. Res. Commun., 121(3): 848–854.
Cafieri F, Fattorusso E, Gavagnin M, Santacroce C. 1985. 3,5,6 -Trihydroxysterols from the Mediterranean bryozoan Myriapora truncata. J. Nat. Prod., 48(6): 944–947.
Dai M C, Tabacchi R, Saturnin C. 1993. Nitrogen-containing aromatic compound from the culture medium of Penicillium chrysogenum Thom. Chimia, 47(6): 226–229.
de la Campa R, Seifert K, Miller J D. 2007. Toxins from strains of Penicillium chrysogenum isolated from buildings and other sources. Mycopathologia, 163(3): 161–168.
Du L, Li D H, Zhu T J, Cai S X, Wang F P, Xiao X, Gu Q Q. 2009. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron, 65(5): 1 033–1 039.
Franzén J, Fisher A. 2009. Asymmetric alkaloid synthesis: a one-pot organocatalytic reaction to quinolizidine derivatives. Angew. Chem. Int. Ed. Engl., 48(4): 787–791.
Gao S S, Li X M, Zhang Y, Li C S, Cui C M, Wang B G. 2011. Comazaphilones A F, azaphilone derivatives from the marine sediment-derived fungus Penicillium commune QSD-17. J. Nat. Prod., 74(2): 256–261.
Haefner B. 2003. Drugs from the deep: marine natural products as drug candidates. Drug Discov. Today, 8(12): 536–544.
Hikino H, Nabetani S, Takemoto T. 1973. Structure and biosynthesis of chrysogine, a metabolite of Penicillium Chrysogenum. Yakugaku Zasshi, 93(5): 619–623.
Kettering M, Sterner O, Anke T. 2004. Antibiotics in the chemical communication of fungi. Z. Naturforsch. C, 59(11): 816–823.
Kozlovsky A G, Zhelifonova V P, Antipova T V, Adanin V M, Novikova N D, Deshevaia E A, Schlegel B, Dahse H M, Gollmick F A, Grafe U. 2004. Penicillium expansum, a resident fungal strain of the orbital complex Mir, producing xanthocillin X and questiomycin A. Prikl. Biokhim. Mikrobiol., 40(3): 344–349.
Larsen T O, Smedsgaard J, Lund F, Frisvad J C, Gareis M. 2000. Chemical characterisation of cheese associated fungi. Mycotoxin Research, 16(1): 109–112.
Miyaji K, Ishiwata N, Nakamura T, Nishino T, Kamiya H, Yamamoto M. 2005. Thrombopoietin receptor activaor and process for producing the same. United States Patent (US 2005/0282730 A1).
Noda N, Kubota S, Miyata Y, Miyahara K. 2000. Optically active N-acetyldopamine dimer of the crude drug “Zentai”, the cast-off shell of the cicada, Cryptotympana sp. Chem. Pharm. Bull., 48(11): 1 749–1 752.
Rahaim Jr, Ronald J, Maleczka Jr, Robert E. 2006. Palladiumcatalyzed silane/siloxane reductions in the one-pot conversion of nitro compounds to their amines, hydroxylamines, amides, sulfonamides, and carbamates. Synthesis, 2006(19): 3 316–3 340.
Rothe W. 1950. Antibiotic complex produced by Penicillium notatum Westling. Pharmazie, 5: 190.
Shen C C, Syu W J, Li S Y, Lin C H, Lee G H, Sun C M. 2002. Antimicrobial activities of naphthazarins from Arnebia euchroma. J. Nat. Prod., 65(12): 1 857–1 862.
Smith W B. 1977. The carbon-13 spectra of steroids on the way to ecdysone. Org. Magn. Reson., 9(11): 644–648.
TimTec Compound Libraries. 2010. Harmony Business Park Building, Newark, USA.
Vesonder R F. 1979. Xanthocillin, a metabolite of Eupenicillium egyptiacum NRRL 1022. J. Nat. Prod., 42(2): 232–233.
Wagener R E, Davis N D, Diener U L. 1980. Penitrem A and roquefortine production by Penicillium commune. Appl. Environ. Microbiol., 39(4): 882–887.
Wang S, Li X M, Teuscher F, Li D L, Diesel A, Ebel R, Proksch P, Wang B G. 2006. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod., 69(11): 1 622–1 625.
Yamaguchi T, Miyake Y, Miyamura A, Ishiwata N, Tatsuta K. 2006. Structure-activity relationships of xanthocillin derivatives as thrombopoietin receptor agonist. J. Antibiot., 59(11): 729–734.
Yue J M, Chen S N, Lin Z W, Sun H D. 2001. Sterols from the fungus Lactarium volemus. Phytochemistry, 56(8): 801–806.
Zhang Y, Wang S, Li X M, Cui C C, Feng C, Wang B G. 2007. New sphingolipids with a previously unreported 9-methyl-C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids, 42(8): 759–764.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the Ministry of Science and Technology (No. 2010CB833802), the Knowledge Innovation Program of Chinese Academy of Sciences (No. KSCX2-EW-G-12B), and the National Natural Science Foundation of China (No. 30910103914)
Rights and permissions
About this article
Cite this article
Shang, Z., Li, X., Meng, L. et al. Chemical profile of the secondary metabolites produced by a deep-sea sediment-derived fungus Penicillium commune SD-118. Chin. J. Ocean. Limnol. 30, 305–314 (2012). https://doi.org/10.1007/s00343-012-1075-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-012-1075-1