Abstract
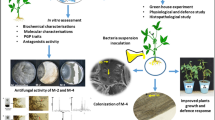
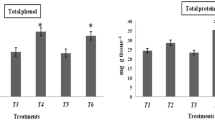
In the present research, bacterial-mediated elicitation of induced systemic resistance in the soybean plant was studied. The main objective was in vitro analysis of jasmonic acid and different defense-related enzymes in soybean plants primed with bacterium Bacillus sp. SJ-5 against the fungal pathogen Rhizoctonia solani and Fusarium oxysporum. In the different assays conducted, Bacillus sp. SJ-5 showed strong antifungal activity against R. solani and F. oxysporum showing 45 and 63 % growth inhibition, respectively. Strain SJ-5 was found to be positive for the cell wall-degrading enzymes chitinase, protease, and β-1,3-glucanase, and cell-free supernatant was found with significant fungal growth inhibitory activity. Different defense-related enzymes, namely lipoxygenase, phenylalanine ammonia-lyase, peroxidase, polyphenol oxidase, and β-1,3-glucanase in the different parts of Glycine max L. Merrill were reported to be highest on the 8th day after challenge inoculation and expressed significantly in the root tissue. GC–MS analysis of jasmonic acid (JA) revealed the highest JA accumulation in bacterized soybean plant root tissue challenged with R. solani and F. oxysporum, which was 91.2 and 99.84 %, respectively, with respect to control. In the SJ-5-primed root tissue, phenolic content was highest upon challenge inoculation of R. solani and F. oxysporum with 30.47 ± 0.97 and 32.4 ± 0.3 mg/g fresh weight, respectively. In summary, the present investigation revealed a role for the bacterial isolate Bacillus sp. SJ-5 in soybean plant growth promotion and enhanced protection against R. solani and F. oxysporum by elicitation of defense-related enzymes.






Similar content being viewed by others
References
Abdel-Monaim MF, Mamdoh Ewis Ismail ME, Morsy KM (2012) Induction of systemic resistance in soybean plants against Fusarium wilts disease by seed treatment with benzothiadiazole and humic acid. Afr J Biotechnol 11(10):2454–2465
Agrawal T, Kotasthane AS (2012) Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. SpringerPlus 1(1):73
Akram W, Mahboob A, Javed A (2013) Bacillus thuringiensis strain 199 can induce systemic resistance in tomato against Fusarium wilt. Eur J Microbiol Immunol 3(4):275–280
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol 24(1):1
Baysal T, Demirdoven A (2007) Lipoxygenase in fruits and vegetables: a review. Enzyme Microb Technol 40(4):491–496
Benhamou N, Gagne S, Quere DL, Dehbi L (2000) Bacterial mediated induced resistance in cucumber: beneficial effect of the endophytic bacterium Serratia plymuthica on the protection against infection by Pythium ultimum. Phytopathol 90:45–56
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Bernard E, Larkin RP, Tavantzis S, Erich MS, Alyokhin A, Sewell G, Lannan A, Gross SD (2012) Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl Soil Ecol 52:29–41
Blechert S, Brodschelm W, Hölder S, Kammerer L, Kutchan TM, Mueller MJ, Xia ZQ, Zenk MH (1995) The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA 92(10):4099–4105
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Browse J (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60:183–205
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Vie´gas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt stressed cowpea leaves. New Phytol 163:563–571
Chang WT, Chen YC, Jao CL (2007) Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour Techol 98:1224–1230
Choudhary DK (2011) Plant growth-promotion (PGP) activities and molecular characterization of rhizobacterial strains isolated from soybean (Glycine max L. Merril) plants against charcoal rot pathogen Macrophomina phaseolina. Biotechnol Lett 33:2287–2295
Compant S, Duffy B, Nowak J, Clement C, Barka AE (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Dodd IC, Perez-Alfocea F (2012) Microbial alleviation of crop salinity stress. J Exp Bot 63:3415–3428
Doornbos RF, Geraats BP, Kuramae EE, Van Loon LC, Bakker PA (2011) Effects of jasmonic acid, ethylene, and salicylic acid signaling on the rhizosphere bacterial community of Arabidopsis thaliana. Mol Plant Microbe Interact 24(4):395–407
Egamberdieva D, Kucharova Z, Davranov K, Berg G, Makarova N, Azarova T, Chebotar V, Tikhonovich I, Kamilova F, Validov SZ, Lugtenberg B (2011) Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol Fertil Soils 47(2):197–205
El-Abady MI, Seadh SE, Attia AN, El-Saidy Aml EA (2008) Impact of foliar fertilization and its time of application on yield and seed quality of soybean. In: The 2th field crops conference, FCRI, AV, Giza, Egypt, 14–16 Oct 2008
Ellis C, Karafyllidis L, Turner JG (2002) Constitutive activation of jasmonate signalling in Arabidopsis mutant correlates with enhanced resistance to Eryshiphe cichoracearum, Pseudomonas syringae and Myzus persicae. Mol Plant Microbe Interact 15:1025–1030
Epple P, Apel K, Bohlmann H (1995) An Arabidopsis thaliana thionin gene is inducible via a signal transduction pathway different from that for pathogenesis-related proteins. Plant Physiol 109:813–820
García-Gutiérrez L, Zeriouh H, Romero D, Cubero J, Vicente A, Pérez-García A (2013) The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate-and salicylic acid-dependent defence responses. Microb Biotechnol 6(3):264–274
Glazebrook J (2001) Genes controlling expression of defense responses in Arabidopsis: 2001 status. Curr Opin Plant Biol 4:301–308
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotech Adv 28(3):367–374
Glick BR (2015) Stress control and ACC deaminase. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer International Publishing, Switzerland, pp 257–264
Govindappa M, Rai VR, Lokesh S (2014) Induction of resistance against Cercospora leaf spot in safflower by seed treatment with plant growth-promoting rhizobacteria. Arch Phytopathol Plant Prot 47(20):2479–2492
Gu XC, Chen JF, Xiao Y, Di P, Xuan HJ, Zhou X, Zhang L, Chen WS (2012) Overexpression of allene oxide cyclase promoted tanshinone/phenolic acid production in Salvia miltiorrhiza. Plant Cell Rep 31(12):2247–2259
Gul A, Ozaktan H, Kidoglu F, Tuzel Y (2013) Rhizobacteria promoted yield of cucumber plants grown in perlite under Fusarium wilt stress. Sci Hortic 153:22–25
Heidari M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soci Agric Sci 11(1):57–61
Hiscox JT, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57(12):1332–1334
Jain S, Vaishnav A, Kasotia A, Kumari S, Gaur RK, Choudhary DK (2014) Rhizobacterium-mediated growth promotion and expression of stress enzymes in Glycine max L. Merrill against Fusarium wilt upon challenge inoculation. World J Microbiol Biotechnol 30(2):399–406
Jaiti F, Verdeil JL, El Hadrami I (2009) Effect of jasmonic acid on the induction of polyphenoloxidase and peroxidase activities in relation to date palm resistance against Fusarium oxysporum f. sp. albedinis. Physiol Mol Plant Pathol 74:84–90
Jayaraj J, Muthukrishnan S, Liang GH, Velazhahan R (2004) Jasmonic acid and salicylic acid induce accumulation of β-1, 3-glucanase and thaumatin-like proteins in wheat and enhance resistance against Stagonospora nodorum. Biol Planta 48(3):425–430
Jeong MJ, Choi BS, Bae DW, Shin SC, Park SU, Lim HS, Kim J, Kim JB, Cho BK, Bae H (2012) Differential expression of kenaf phenylalanine ammo nia-lyase (PAL) ortholog during developmental stages and in response to abiotic stresses. Plant Omics 5(4):392–399
Jiang K, Pi Y, Hou R, Jiang L, Sun X, Tang K (2009) Promotion of nicotine biosynthesis in transgenic tobacco by overexpressing allene oxide cyclase from Hyoscyamus niger. Planta 229(5):1057–1063
Kavitha K, Mathiyazhagan S, Senthilvel V, Nakkeeran S, Chandrasekar G (2005) Development of bioformulations of antagonistic bacteria for the management of damping off of chilli (Capsicum annuum L.). Arch Phytopathol Plant Prot 38:19–30
Kramell R, Miersch O, Atzorn R, Parthier B, Wasternack C (2000) Octadecanoid-derived alteration of gene expression and the “oxylipin signature” in stressed barley leaves. Implications for different signaling pathways. Plant Physiol 123(1):177–188
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167:493–499
Lee BK, Park MR, Srinivas B, Chun JC, Kwon IS, Chung IM, Yoo NH, Choi KG, Yun SJ (2003) Induction of phenylalanine ammonia-lyase gene expression by paraquat and stress-related hormones in Rehmannia glutinosa. Mol Cells 16(1):34–39
Liang X, Dron M, Schmid J, Dixon R, Lamb C (1989) Developmental and environmental regulation of a phenylalanine ammonia-lyase-b-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci USA 86:9284–9288
Liu W, Mazarei M, Rudis MR, Fethe MH, Peng Y, Millwood RJ, Schoene G, Burris JN, Stewart CN (2013) Bacterial pathogen phytosensing in transgenic tobacco and Arabidopsis plants. Plant Biotechnol J 11(1):43–52
Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signaling network. Curr Opin Plant Biol 8:532–540
Lu ZX, Gaudet D, Puchalski B, Despins T, Frick M, Laroche A (2006) Inducers of resistance reduce common bunt infection in wheat seedlings while differentially regulating defence-gene expression. Physiol Mol Plant Pathol 67:138–148
Ludueña LM, Taurian T, Tonelli ML, Angelini JG, Anzuay MS, Valetti L, Muñoz V, Fabra AI (2012) Biocontrol bacterial communities associated with diseased peanut (Arachis hypogaea L.) plants. Eur J Soil Biol 53:48–55
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63:541–556
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258
Mayer AM, Harel E (1979) Polyphenol oxidases in plants. Phytochem 18:193–215
McDonald S, Prenzler PD, Antolovich M, Robards K (2001) Phenolic content and antioxidant activity of olive extracts. Food Chem 73(1):73–84
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mori T, Sakurai M, Sakuta M (2001) Effects of conditioned medium on activities of PAL, CHS, DAHP synthase (DS-Co and DS-Mn) and anthocyanin production in suspension cultures of Fragaria ananassa. Plant Sci 160:355–360
Nadeem SM, Zahir ZA, Naveed M, Asghar HN, Arshad M (2010) Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci Soc Am J 74(2):533–542
Pan SQ, Ye XS, Kuć J (1989) Direct detection of beta-1,3-glucanase isozymes on polyacrylamide electrophoresis and isoelectrofocusing gels. Anal Biochem 182(1):136–140
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Samblanx GW, Buchala A, Métraux J-P, Manners JM, Broekaert WF (1996) Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8:2309–2323
Pieterse CMJ, Ton J, Van Loon LC (2001) Cross-talk between plant defence signalling pathways: boost or burden? AgBiotechNet 3:1–8
Pineda A, Soler R, Weldegergis BT, Shimwela MM, Van Loon JJ, Dicke M (2013) Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via Jasmonic acid signaling. Plant Cell Environ 36:393–404
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil 239:55–68
Reyes-Ramirez A, Escudero-Abarca BI, Aguilar-Uscanga G, Hayward-Jones PM, Barboza-Corona JE (2004) Antifungal activity of Bacillus thuringiensis chitinase and its potential for the biocontrol of phytopathogenic fungi in soybean seeds. J Food Sci 69:131–134
Rosahl S (1996) Lipoxygenases in plants: their role in development and stress response. Z Naturforsch C 51:123–138
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169:18–29
Saravitz DM, Siedow JN (1996) The differential expression of wound-inducible lipoxygenase genes in soybean leaves. Plant Physiol 110:287–299
Schweizer P, Buchala A, Silverman P, Seskar M, Raskin I, Metraux JP (1997) Jasmonate-inducible genes are activated in rice by pathogen attack without a concomitant increase in endogenous jasmonic acid levels. Plant Physiol 114(1):79–88
Senthilraja G, Anand T, Kennedy JS, Raguchander T, Samiyappan R (2013) Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leaf miner insect and collar rot pathogen. Physiol Mol Plant Pathol 82:10–19
Seo DJ, Nguyen DMC, Song YS, Jung WJ (2012) Induction of defense response against Rhizoctonia solani in Cucumber plants by endophytic bacterium Bacillus thuringiensis GS1. J Microbiol Biotechnol 22(3):407–415
Shah J (2005) Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu Rev Phytopathol 43:229–260
Solanki MK, Kumar S, Pandey AK, Srivastava S, Singh RK, Kashyap PL, Srivastava AK, Arora DK (2012) Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocontrol Sci Technol 22:203–217
Sundaramoorthy S, Raguchander T, Ragupathi N, Samiyappan R (2012) Combinatorial effect of endophytic and plant growth promoting rhizobacteria against wilt disease of Capsicum annum L. caused by Fusarium solani. Biol Control 60(1):59–67
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5(1):51–58
Thomma BPHJ, Penninckx IAMA, Cammue BPA, Broekaert WF (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13:63–68
Van Wees SCM, Van der Ent S, Pieterse CMJ (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11:443–448
Vick BA, Zimmerman DC (1984) Biosynthesis of jasmonic acid by several plant species. Plant Physiol 2:458–461
Vijayan P, Shockey J, Lévesque A, Cook RJ, Browse J (1998) A role for jasmonates in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95:7209–7214
Vincent JM (1947) Distortion of fungal hyphae in the presence ofcertain inhibitors. Nature 159:850
Wahyudi AT, Astuti RP, Widyawati A, Meryandini A, Nawangsih AA (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting Rhizobacteria. J Microbiol Antimicrob 3(2):34–40
Walia A, Mehta P, Chauhan A, Shirkot CK (2013) Antagonistic Activity of plant growth promoting rhizobacteria isolated from tomato rhizosphere against soil borne fungal plant pathogens. Int J Agric Environ Biotechnol 6(4):571–580
Wang SL, Hsiao WJ, Chang WT (2002) Purification and characterization of an antimicrobial chitinase extracellularly produced by Monascus purpureus CCRC31499 in a shrimp and crab shell powder medium. J Agric Food Chem 50:2249–2255
Weller DM (1988) Biological control of soil borne plant pathogens in rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407
Yang DH, Hettenhausen C, Baldwin IT, Wu JQ (2011) BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata’s responses to herbivory. J Exp Bot 62:641–652
Acknowledgments
The research was supported by DBT Grant No. BT/PR1231/AGR/21/340/2011 to DKC. Some of the research has been supported by SERB-DST Grant No. SR/FT/LS-129/2012 to DKC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, S., Vaishnav, A., Kumari, S. et al. Chitinolytic Bacillus-Mediated Induction of Jasmonic Acid and Defense-Related Proteins in Soybean (Glycine max L. Merrill) Plant Against Rhizoctonia solani and Fusarium oxysporum . J Plant Growth Regul 36, 200–214 (2017). https://doi.org/10.1007/s00344-016-9630-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9630-1