Abstract
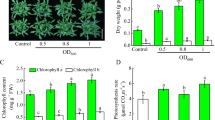
We investigated the effects of microbial volatile organic compounds (mVOC) emitted by Bacillus amyloliquefaciens GB03 on Mentha piperita growing under different levels of NaCl stress, by evaluating their growth-promoting potential and ability to increase salt tolerance effects. Plants were exposed to bacterial VOCs without having any physical contact with the rhizobacteria. The VOCs emitted by the rhizobacteria (mVOCs) were analyzed using SPME fibers. An increase in the level of salt concentration led to a decrease in plant growth. However, these negative effects of salinity were inhibited in the plants exposed to mVOCs. Plants grown in a saline media and exposed to GB03 VOCs had significantly better morphological characteristics and higher total chlorophyll content compared to controls. The level of endogenous jasmonic acid (JA), salicylic acid, and abscisic acid increased in salt-stressed plants compared to controls. The level of JA did not show any change in plants grown in a saline media either exposed to mVOCs or not. In contrast, the amount of salicylic acid increased remarkably in salt-stressed plants exposed to mVOCs compared to controls (salt-stressed plants not exposed to mVOCs), but the levels of abscisic acid decreased in salt-stressed plants exposed to mVOCs. The chromatographic analyses of the mVOCs produced by salt-stressed GB03 bacteria were similar, regardless of the concentration of salt in the media where the bacteria were grown, although it was observed that the relative percentage of acetoin increased with salt concentration. After determining that acetoin was the main VOCs compound, we exposed plants to acetoin, which demonstrated that acetoin caused similar effects on plants grown under salt stress conditions as those exposed to GB03 mVOCs. Based on these results, the use of mVOCs from PGPR is suggested as a useful technological innovation to facilitate the growth of M. piperita in salt-stressed environments.




Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- JA:
-
Jasmonic acid
- MS:
-
Murashige and Skoog medium
- PGPR:
-
Plant-growth-promoting rhizobacteria
- SA:
-
Salicylic acid
References
Abogadallah GM (2010) Antioxidative defense under salt stress. Plant Signal Behav 5:369–374
Ann MN, Cho YE, Ryu HJ, Kim HT, Park KS (2013) Growth promotion of tobacco plant by 3-hydroxy-2-butanone from Bacillus vallismortis EXTN-1. Korean J Pestic Sci 17:388–393. https://doi.org/10.7585/kjps.2013.17.4.388
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Banchio E, Xie X, Zhang H, Paré PW (2009) Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J Agric Food Chem 57:653–657
Bertani G (1951) Studies on lysogenesis. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300
Bharti N, Barnawal D, Awasthi A, Yadav A, Kalra A (2014) Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Mentha arvensis. Acta Physiol Plant 36:45–60
Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, Eberl L, Weisskopf L (2011) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058
Cappellari LR, Santoro MV, Reinoso H, Travaglia C, Giordano W, Banchio E (2015) Anatomical, morphological, and phytochemical effects of inoculation withplant growth- promoting rhizobacteria on peppermint (Mentha piperita). J Chem Ecol 41:149–158. https://doi.org/10.1007/s10886-015-0549-y
Chakraborty U, Chakraborty BN, Chakraborty AP, Dey PL (2013) Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J Microbiol Biotechnol 29:789–803
Cho SM, Kang BR, Han SH, Anderson AJ, Park JY, Lee YH, Cho BH, Yang KY, Ryu CM, Kim YC (2008) 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabdopsis thaliana. Mol Plant-Microbe Interact 21:1067–1075
Choi SK, Jeong H, Kloepper JW, Ryu CM (2014) Genome sequence of Bacillus amyloliquefaciens GB03, an active ingredient of the first commercial biological control product. Gen Announc 2:01092–01098
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Farag MA, Ryu CM, Sumner LW, Pare PW (2006) GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochem 67:2262–2268
Farag MA, Zhang H, Ryu CM (2013) Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J Chem Ecol 39:1007–1018
Fernando VCD, Schroeder DF (2016) Role of ABA in Arabidopsis salt, drought, and desiccation tolerance. In: Shanker AK, Shanker C (eds) Abiotic and biotic stress in plants - recent advances and future perspectives. InTech, Rijeka- Croatia
Fincheira P, Quiroz A (2018) Microbial volatiles as plant growth inducers. Microbiol Res 208:63–75
Fincheira P, Venthur H, Mutis A, Parada M, Quiroz A (2016) Growth promotion of Lactuca sativa in response to volatile organic compounds emitted from diverse bacterial species. Microbiol Res 193:39–47
Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:15–45
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768
Jin CW, Ye YQ, Zheng SJ (2014) An underground tale: contribution of microbial activity to plant iron acquisition via ecological processes. Ann Bot 113:7–18
Kang SM, Khana AL, Waqasa M, You YH, Kim JH, Kim JG et al (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9:673–682. https://doi.org/10.1080/17429145.2014.894587
Khan MI, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462
Kim K, Jang YJ, Lee SM, Oh BT, Chae JC, Lee KJ (2014) Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol Cells 37:109–117
Kloepper JW (1993) Plant growth promoting rhizobacteria as biological control agents. In: Meeting FB Jr (ed) Soil microbial ecology-applications in agricultural and environmental management. Marcel Dekker, New York, pp 255–274
Kuan KB, Othman R, Rahim AK, Shamsuddin ZH (2016) Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 11:e0152478
Ledger T, Rojas S, Timmermann T, Pinedo I, Poupin MJ, Garrido T, Richter P, Tamayo J, Donoso R (2016) Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front Microbiol 7:1838
Li J, Jia H, Wang J (2014) cGMP and ethylene are involved in maintaining ion homeostasis under salt stress in Arabidopsis roots. Plant Cell Rep 33:447-459. https://doi.org/10.1007/s00299-013-1545-8
Li Z, Yang H, Wu X, Guo K, Li J (2015) Some aspects of salinity responses in peppermint (Mentha × piperita L.) to NaCl treatment. Protoplasma 252:885–899
Liu XM, Zhang H (2015) The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front Plant Sci 6:774
Mimouni H, Wasti S, Manaa A, Gharbi E, Chalh A, Vandoorne B, Lutts S, Ahmed HB (2016) Does salicylic acid (SA) improve tolerance to salt stress in plants? a study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. OMICS 20(3):180-190
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 5:473–497
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11
Rudrappa T, Biedrzycki ML, Kunjeti SG, Donofrio NM, Czymmek KJ, Paré PW, Bais HP (2010) The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun Integr Biol 3:130–138
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper J (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Salehi B, Stojanović-Radić Z, Matejić J, Sharopov F, Antolak H, Kręgiel D, Sen S, Sharifi-Rad M, Acharya K, Sharifi-Rad R, Martorell M, Sureda A, Martins N, Sharifi-Rad J (2018) Plants of genus Mentha: from farm to food factory. Plants 7(3):70. https://doi.org/10.3390/plants7030070
Santoro M, Zygadlo J, Giordano W, Banchio E (2011) Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol Biochem 49:1077–1082
Santoro MV, Bogino PC, Nocelli N, del Cappellari L, Giordano WF, Banchio E (2016) Analysis of plant growth-promoting effects of fluorescent pseudomonas strains isolated from mentha piperita rhizosphere and effects of their volatile organic compounds on essential oil composition. Front Microbiol 7:1085
Schmidt A, Nagel R, Krekling T, Christiansen E, Gershenzon J, Krokene P (2011) Induction of isoprenyl diphosphate synthases, plant hormones and defense signalling genes correlates with traumatic resin duct formation in Norway spruce (Picea abies). Plant Mol Biol 77:577–590
Subramanian K, Charest C (1997) Nutritional, growth, and reproductive responses of maize (Zea mays L.) to arbuscular mycorrhizal inoculation during and after drought stress at tasseling. Mycorrhiza 7:25
Vaishnav A, Kumari S, Jain S, Varma A, Choudhary DK (2015) Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J Appl Microbiol 119:539–551
Valenzuela CE, Acevedo-Acevedo O, Miranda GS, Vergara-Barros P, Holuigue L, Figueroa CR, Figueroa PM (2016) Salt stress response triggers activation of the jasmonate signaling pathway leading to inhibition of cell elongation in Arabidopsis primary root. J Exp Bot 67:4209–4220
Van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, dit Frey NF, Leung J (2008) An update on abscisic acid signalling in plants and more. Mol Plant 1:198–217
Wenke K, Weise T, Warnke R, Valverde C, Wanke D, Kai M, Pichuella B (2012) Bacterial volatiles mediating information between bacteria and plants. In: Witzany G (ed) Biocommunication, signaling and communication in plants. Springer, Berlin, pp 327–347
Wintermans PC, Bakker PA, Pieterse CM (2016) Natural genetic variation in Arabidopsis for responsiveness to plant growth-promoting rhizobacteria. Plant Mol Biol 90:623–634
Yang T, Zhiming R, Zhang X, Xu M, Xu Z, Yang ST (2017) Metabolic engineering strategies for acetoin and 2,3-butanediol production: advances and prospects. Crit Rev Biotechnol 37:990–1005
Zhang H, Kim MS, Krishnamchari V, Payton P, Sun Y, Grimson M, Frag MA, Ryu CM, Allen R, Melo IS, Pare PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Zhang H, Kim MS, Sun Y, Dowd SE, Shi H, Paré PW (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant-Microbe Interact 21:737–744
Zhang H, Sun Y, Xie X, Kim MS, Dowd SE, Paré PW (2009) A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J 58:568–577
Zhang H, Murzello C, Sun Y, Kim MS, Xie X, Jeter RM, Zak JC, Dowd SE, Paré PW (2010) Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol Plant Microbe Interact 23:1097–1104
Zhao Y, Xing L, Wang X, Hou YJ, Gao J, Wang P, Duan CG, Zhu X, Zhu JK (2014) The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci Signal 7(328):ra53
Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4:401–406
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgements
This research was supported by Grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT). EB is a Career Member of CONICET. LC has fellowships from CONICET. The authors are grateful to Dr. Paul Hobson, native speaker, for editorial assistance.
Funding
This study was supported by Grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), MinCyT Córdoba, the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina, and financial support to EB from the Georg Forster Research Fellowship of the Alexander von Humboldt Foundation. EB is a Career Member of CONICET. LC and MVS received fellowships from CONICET- Min CyT.
Author information
Authors and Affiliations
Contributions
LC performed the experiments, and EB designed the research and analyzed the data. EB and LC wrote the manuscript. All the authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cappellari, L.d.R., Banchio, E. Microbial Volatile Organic Compounds Produced by Bacillus amyloliquefaciens GB03 Ameliorate the Effects of Salt Stress in Mentha piperita Principally Through Acetoin Emission. J Plant Growth Regul 39, 764–775 (2020). https://doi.org/10.1007/s00344-019-10020-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10020-3