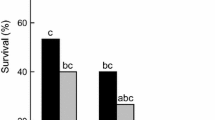
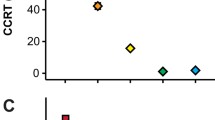
Abstract
The strategy for cold-hardiness and water balance features of two closely related families of Coleoptera, Cerambycidae and Chrysomelidae, were investigated. Cerambycids were freeze-avoiding with low supercooling points, whereas chrysomelids froze at high temperatures and were tolerant to freezing. Hence, the two families have adopted different strategies for cold-hardiness. Due to their low trans-cuticular water permeability, the cerambycids have low rates of evaporative water loss. Chrysomelids have much higher trans-cuticular water permeability, but freezing brings their body fluids in vapour pressure equilibrium with ice and prevents evaporative water loss. The differences in cold-hardiness strategies and rates of water loss are likely to reflect the water content of the diets of the two families. Cerambycids feed on dry wood with low water content, causing a restrictive water balance. Chrysomelids feed on leaves with high water content and may use evaporation through the cuticle as a route of water excretion. Haemolymph ice nucleators help chrysomelids to freeze at a high temperature and thus to maximize the period they spend in the water saving frozen state. The diet-related differences in water balance may be the reason why the two families have developed different strategies for cold-hardiness.

Similar content being viewed by others
Abbreviations
- AFP:
-
Antifreeze protein
- FRWL:
-
Fraction of respiratory water loss.
- FTCWL:
-
Fraction of trans-cuticular water loss
- HFP:
-
Hysteresis freezing point
- INA:
-
Ice nucleating agent
- M:
-
Metabolic rate
- MP:
-
Melting point
- RWL:
-
Rate of respiratory water loss
- TWL:
-
Rate of total water loss
References
Bailey NTJ (1974) Statistical Methods in Biology. Unibooks, English Universities Press, London, 200 pp
Dissanayake P, Zachariassen KE (1980) Effect of warm acclimation on the cationic concentrations in the extracellular and intracellular body fluid of hibernating Rhagium inquisitor beetles. Comp Biochem Physiol A 65:347–350
Engelmann MD (1963) A constant pressure respirometer for small arthropods. Entomol News 74:181–187
Evans LS, Ting IP (1974) Ozone sensitivity of leaves: relationship to leaf water content, gas transfer resistance and anatomical characteristics. Am J Bot 61:592–597
Jeuniaux C (1971) Hemolymph—Arthropoda. In: Florkin H, Scheer BT (eds) Chemical Zoology, vol VI. Academic Press, New York, pp 63–118
van der Laak S (1982) Physiological adaptations to low temperature in freezing tolerant Phyllodecta laticollis beetles. Comp Biochem Physiol A 73:613–620
Linssen EF (1959) Beetles of the British Isles II. Frederick Warne & Co. Ltd., London, 295 p
Lundheim R, Zachariassen KE (1993) Water balance of over-wintering beetles in relation to strategies for cold tolerance. J Comp Physiol B 163:1–4
Miller LK (1982) Cold-hardiness strategies of some adult and immature insects overwintering in interior Alaska. Comp Biochem Physiol A 73:595–604
Ødegaard F, Ramløv H, Zachariassen KE (2003) Thermal hysteresis, cold stress and species distribution. In: Ivanov B, Maximov T (eds) Influence of climatic and ecological changes on permafrost ecosystems. Publishing House of the Siberian Division of the Russian Academy of Sciences, Yakutsk, pp 190–193
Olsen TM, Duman JG (1997a) Maintenance of the supercooled state in the overwintering pyrochroid beetle larvae, Dendroides canadensis: role of hemolymph ice nucleators and antifreeze proteins. J Comp Physiol B 167:105–113
Olsen TM, Duman JG (1997b) Maintenance of the supercooled state in the gut fluid of overwintering pyrochroid beetle larvae, Dendroides canadensis: role of ice nucleators and antifreeze proteins. J Comp Physiol B 167:114–122
Ramløv H (2000) Aspects of natural cold tolerance in ectothermic animals. Hum Rep 15:26–46
Ring R (1982) Freezing-tolerant insects with low supercooling points. Comp Biochem Physiol A 73:605–612
Røskaft E, Zachariassen KE, Maloiy GMO, Kamau JMZ (1986) Temperature regulation and water balance of day-active Zophosis congesta beetles in East Africa. J Trop Ecol 2:139–146
Salt RW (1961) Principles of insect cold hardiness. Ann Rev Entomol 6:55–74
Sinclair BJ (1999) Insect cold tolerance: how many kinds of frozen? Eur J Entomol 96:157–164
Sømme L, Conradi-Larsen EM (1979) Frost resistance in alpine, adult Melasoma collaris (Coleoptera). Oikos 33:80–84
Sømme L, Zachariassen KE (1981) Adaptations to low temperatures in high altitude insects from Mount Kenya. Ecol Entomol 6:199–204
Storey KB, Storey JM (1986) Freeze-tolerance and intolerance as strategies of winter survival of terrestrially-hibernating amphibians. Comp Biochem Physiol A 83:613–617
Sutcliffe DW (1963) The chemical composition of haemolymph in insects and some other arthropods in relation to their phylogeny. Comp Biochem Physiol 9:121–135
Voituron Y, Mouguet N, de Mazancourt C, Claubert J (2002) To freeze or not to freeze? An evolutionary perspective on the hold-hardiness strategies of overwintering ectotherms. Am Nat 160:255–270
Zachariassen KE (1979) The mechanism of the cryoprotective effect of glycerol in beetles tolerant to freezing. J Insect Physiol 25:29–32
Zachariassen KE (1980) The role of polyols and nucleating agents in cold-hardy beetles. J Comp Physiol 140:227–234
Zachariassen KE (1991) Routes of transpiratory water loss in a dry habitat tenebrionid beetle. J Exp Biol 157:425–437
Zachariassen KE, Hammel HT (1976) Nucleating agents in the haemolymph of insects tolerant to freezing. Nature 262:285–287
Zachariassen KE, Kristiansen E (2000) Ice nucleation and antinucleation in nature. Cryobiology 41:257–279
Zachariassen KE, Maloiy GMO (1989) Water balance of beetles as an indicator of environmental humidity. Fauna Norvegica B 36:27–31
Zachariassen KE, Baust JG, Lee RE Jr (1982) A method for quantitative determination of ice nucleating agents in insect hemolymph. Cryobiology 19:180–184
Zachariassen KE, Andersen J, Maloiy GMO, Kamau GMZ (1987) Transpiratory water loss and metabolism of beetles from arid areas in East Africa. Comp Biochem Physiol A 68:403–408
Zachariassen KE, Kristiansen E, Pedersen SA, Hammel HT (2004a) Ice nucleation in solutions and freeze-avoiding insects–homogeneous or heterogeneous? Cryobiology 48:309–321
Zachariassen KE, Kristiansen E, Pedersen SA (2004b) Inorganic ions in cold-hardiness. Cryobiology 48:126–133
Acknowledgments
The present study was supported by the Norwegian Research Council (NRF) over grant no. 1167106/V40 to Karl Erik Zachariassen. The authors would like to thank Christer Reiråskag for help with collecting the beetles. The present experiments comply with the “Principles of Animal Care”, publication No. 86-23, revised 1985 of the National Institute of Health and also with the current laws of Norway.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Zachariassen, K.E., Li, N.G., Laugsand, A.E. et al. Is the strategy for cold hardiness in insects determined by their water balance? A study on two closely related families of beetles: Cerambycidae and Chrysomelidae. J Comp Physiol B 178, 977–984 (2008). https://doi.org/10.1007/s00360-008-0284-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-008-0284-6