Abstract
Purpose
The aim of current systematic review was to update the body of evidence on associations between adherence to the Mediterranean diet (MedDiet) and risk of cancer mortality, site-specific cancer in the general population; all-cause, and cancer mortality as well as cancer reoccurrence among cancer survivors.
Methods
A literature search for randomized controlled trials (RCTs), case–control and cohort studies published up to April 2020 was performed using PubMed and Scopus. Study-specific risk estimates for the highest versus lowest adherence to the MedDiet category were pooled using random-effects meta-analyses. Certainty of evidence from cohort studies and RCTs was evaluated using the NutriGrade scoring system.
Results
The updated search revealed 44 studies not identified in the previous review. Altogether, 117 studies including 3,202,496 participants were enclosed for meta-analysis. The highest adherence to MedDiet was inversely associated with cancer mortality (RRcohort: 0.87, 95% CI 0.82, 0.92; N = 18 studies), all-cause mortality among cancer survivors (RRcohort: 0.75, 95% CI 0.66, 0.86; N = 8), breast (RRobservational: 0.94, 95% CI 0.90, 0.97; N = 23), colorectal (RRobservational: 0.83, 95% CI 0.76, 0.90; N = 17), head and neck (RRobservational: 0.56, 95% CI 0.44, 0.72; N = 9), respiratory (RRcohort: 0.84, 95% CI 0.76, 0.94; N = 5), gastric (RRobservational: 0.70, 95% CI 0.61, 0.80; N = 7), bladder (RRobservational: 0.87, 95% CI 0.76, 0.98; N = 4), and liver cancer (RRobservational: 0.64, 95% CI 0.54, 0.75; N = 4). Adhering to MedDiet did not modify risk of blood, esophageal, pancreatic and prostate cancer risk.
Conclusion
In conclusion, our results suggest that highest adherence to the MedDiet was related to lower risk of cancer mortality in the general population, and all-cause mortality among cancer survivors as well as colorectal, head and neck, respiratory, gastric, liver and bladder cancer risks. Moderate certainty of evidence from cohort studies suggest an inverse association for cancer mortality and colorectal cancer, but most of the comparisons were rated as low or very low certainty of evidence.
Similar content being viewed by others
Introduction
Cancer is widely recognized as one of the leading public health issues worldwide. According to the GLOBOCAN estimates, in 2018 there were 18.1 million new cases of cancer and it contributed to the death of 9.6 million people [1]. Despite a decreasing trend in cancer mortality observed in recent years, it is still the second most common cause of death world-wide, second to cardiovascular diseases (CVD) [2]. Taking into account trends of recent years, e.g., in Europe and the US, there seems to be a transition concerning the distribution of these two main causes of death. It is reasonable to speculate that cancer will replace CVD as the major cause of death in years to come. According to recent data provided by the PURE study group, this has already happened in a number of high- as well as middle-income countries in adults aged 35–70 years [2]. Until 2040, the global burden of neoplasms is going to rise by more than half [3]. Due to simultaneous improvements in diagnosis and treatment approaches, there will be a substantial increase in the number of cancer survivors as well [4].
Irrespective of site-specific details in the pathogenesis of tumors, up to more than 90% of cancers are considered to be attributable to modifiable risk factors such as tobacco smoking, excessive body weight, physical inactivity, alcohol consumption, infectious agents, environmental pollution, and suboptimal diet [5, 6]. The latter is made responsible for about 5–10% of total cancer cases [5, 7, 8].
According to the World Cancer Research Fund (WCRF), high consumption of fruits, vegetables and whole grains, as well as low intake of red and processed meat can lower cancer risk. As food items and nutrients are consumed in combination, dietary patterns have been successfully implemented as a tool to assess the additive or synergistic effect of food in nutritional epidemiology [9, 10].
With regard to prevention of non-communicable diseases, one of the most well-represented dietary patterns in literature is the Mediterranean diet (MedDiet) [11]. The MedDiet is a plant-based pattern characterized by high amounts of fruits, vegetables, nuts, legumes, fish, cereals including whole grains, and extra-virgin olive oil, at the same time reducing intake of red, processed meat, eggs and dairy [12]. An additional component is a moderate intake of red wine [12]. A large body of clinical and epidemiological studies have observed the protective effect of the MedDiet on cardiovascular disease, diabetes, obesity as well as cancer [13].
We previously conducted a systematic review and meta-analysis on the association between adherence to the MedDiet and risk of cancer, which was followed by two updates [14,15,16]. In the last update, we were able to pool data from 83 studies (including randomized controlled trials, cohort and case–control studies) showing an inverse association between the highest MedDiet adherence category and the risk of cancer mortality as well as incidence of breast, colorectal, gastric, liver, head and neck, and prostate cancer [16]. Although little time has passed, since then, we decided to synthesize the available data in another update due to the following reasons. Since the publication of the latest version of the review, the number of new reports from cohort and case–control studies has increased substantially [17,18,19]. Additionally, some of the new studies focus on cancer subtypes not previously included in our reports [20, 21]. Moreover, we wanted to expand our findings by assessment of the certainty of evidence, which is rarely evaluated in nutrition research evidence syntheses.
Therefore, the aim of this review was to enhance our previous findings on adherence to the MedDiet pattern and risk of cancer mortality, site-specific cancer and all-cause as well as cancer mortality among cancer survivors. Additionally, we aimed to assess the certainty of evidence for identified comparisons.
Methods
The protocol for previous versions of this review was published in PROSPERO International Prospective Register of Systematic Reviews (CRD42013004382). This update of the systematic review was planned and conducted according to the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22].
Search strategy
Two electronic databases, PubMed (from August 2017 to April 2020) and Scopus (from January 2017 to April 2020), were searched with no limitations to publication language. Following search terms were adopted for PubMed and Scopus: (“Mediterranean diet” OR “Mediterranean” OR “diet” OR “dietary pattern” OR “dietary score” OR “dietary adherence”) AND (“cancer” OR “neoplasm” OR “neoplastic disease” OR “survivors” OR “recurrence”) AND (“prospective” OR “follow-up” OR “cohort” OR “longitudinal” OR “case–control”). References from identified articles, systematic reviews and meta-analyses were screened for potential eligibility.
Study selection
Two reviewers (J.M. and A.D.) independently evaluated the eligibility of studies with any disagreements resolved by discussion with the third reviewer (L.S.). In contrast to previous versions of this review, we expanded our analyses with all-cause mortality among cancer survivors. Studies were included if they fulfilled the following criteria: (1) randomized controlled trials (RCTs), prospective cohort, case–cohort, nested case–control, or case–control studies, (2) conducted in adult population (aged ≥ 18 years) which (3) assessed association between adherence to MedDiet and (4) risk of cancer mortality, site-specific cancer, all-cause, cancer mortality or cancer reoccurrence among cancer survivors. If several reports from a single study were available, the one with longer follow-up or a larger number of participants/cases was selected.
Data extraction
After completing selection of eligible studies, two reviewers (J.M. and L.S.) extracted the following data: (1) name of first author, (2) country, (3) study name, (4) study design, (5) outcome, (6) population size, (7) number of cases, (8) length of the study follow-up, (9) age at entry, (10) sex, (11) composition of the MedDiet score and its range, (12) adjustment set and (13) multivariable risk estimates (odds ratio (OR), risk ratio (RR) or hazard ratio (HR) comparing groups of highest and lowest adherence to MedDiet) with corresponding 95% confidence intervals (CI). If a study presented several risk estimates, the one with maximal adjustment was chosen. If separate results for men and women or different cancer subtypes were presented in a study, the estimates were pooled using a fixed-effects model.
Certainty of evidence assessment
To evaluate the certainty of evidence for associations between adherence to MedDiet and cancer outcomes in cohort studies and RCTs, the NutriGrade tool was adopted [23]. This tool is based on the following 9 items: (1) risk of bias, study quality, and study limitations (maximum 2 points for cohorts or 3 points for RCTs); (2) precision (maximum 1 point); (3) heterogeneity (maximum 1 point); (4) directness (maximum 1 point); (5) publication bias (maximum 1 point); (6) funding bias (maximum 1 point); (7) study design (+ 2 points—only for RCTs); (8) effect size (maximum 2 points—only for cohort studies); and (9) dose–response (maximum 1 point—only for cohort studies). Risk of bias domain was assessed using a checklist created by authors of the tool. Four categories based on the total score were used to interpret the certainty of evidence: very low (0 to < 4 points), low (4 to < 6 points), moderate (6 to < 8 points) and high (≥ 8 points).
Statistical analysis
The meta-analysis was conducted by pooling the multivariable-adjusted RRs, HRs or ORs of the highest compared with the lowest MedDiet adherence category using a random-effects model with the DerSimonian–Laird method [24]. Outcomes in the meta-analysis were assumed to be ORs in case-control studies and RRs in prospective studies and RCTs. Using an inverse variance method, the standard error (SE) for the log-transformed OR/RR was calculated and interpreted as an estimated variance of log-transformed OR/RR to weight each study [24]. Included studies were categorized according to the following clinical outcomes: (1) cancer mortality, (2) biliary tract cancer, (3) bladder cancer, (4) blood cancer, (5) breast cancer, (6) colorectal cancer, (7) endometrial cancer, (8) esophageal cancer, (9) gallbladder cancer, (10) gastric cancer, (11) glioma, (12) head and neck cancer, (13) liver cancer, (14) ovarian cancer, (15) pancreatic cancer, (16) prostate cancer, (17) respiratory cancer, (18) skin cancer, (19) all-cause mortality, (20) cancer mortality, and (21) cancer reoccurrence among cancer survivors. Estimates from case–control, cohort studies and RCTs were compared separately. Joint estimates for observational studies were obtained by pooling together data from case–control and cohort studies in the same model. Additional analyses were conducted for associations between individual components of the MedDiet and overall cancer risk:
-
Alcohol (within the range vs. higher consumption)
-
Cereals (higher vs. lower consumption)
-
Dairy (lower vs. higher consumption)
-
Fish (higher vs. lower consumption)
-
Fruit (higher vs. lower consumption)
-
Legumes (higher vs. lower consumption)
-
Meat (lower vs. higher consumption)
-
Nuts (higher vs. lower consumption)
-
Olive oil (higher vs. lower consumption)
-
Vegetables (higher vs. lower consumption)
-
Whole grains (higher vs. lower consumption)
I2 statistic and Cochran’s Q test were used to evaluate the heterogeneity between studies. For the I2 value greater than 50% indicated a substantial statistical heterogeneity [25]. Subgroup analyses were conducted only for prospective cohort studies, for comparisons which included ≥ 10 studies and were stratified for sex (male/female), geographical location (Mediterranean/non-Mediterranean countries) and type of MedDiet score (Trichopoulou MedDiet score [12]/Fung MedDiet score [26]). For breast cancer, pooled risk estimates were additionally compared by menopausal status (premenopausal/postmenopausal) and receptor expression (ER/PR/HER/mixed). Furthermore, analysis for colorectal cancer risk was run separately for anatomical location (proximal colon/distal colon/rectum).
For comparisons with ≥ 10 studies, small-study effects, such as publication bias, were explored by funnel plots and Egger’s regression test, as recommended by Cochrane Collaboration [27]. All analyses were conducted in Review Manager version 5.3 (Nordic Cochrane Center, Copenhagen, Denmark) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) with the “metafor” package [28].
Results
Database search and study characteristics
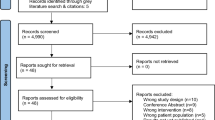
The updated literature search revealed 3720 publications after removal of duplicates from different databases. Additionally, 83 studies identified in previous versions of this systematic review were re-considered [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111]. After title-abstract screening, 137 articles were assessed for eligibility and 20 articles were excluded at this step (ESM Table 1). Details of the study search and selection process were presented as a PRISMA-compliant flowchart in ESM Fig. 1.
Main characteristics of studies identified in the updated search are summarized in Table 1. Overall, 117 studies (with 12 case–control [112,113,114,115,116,117,118,119,120,121,122,123], 26 cohort [17,18,19,20,21, 124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144], five case–cohort [145,146,147,148,149], and one RCT (corrected report) [111] not identified in previous versions of this review) pooling 3,202,496 participants were included in the update [17,18,19,20,21, 29,30,31,32,33,34, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63, 65,66,67,68,69,70,71,72, 80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149].
Definitions of Mediterranean diet
The majority of 44 newly included studies assessed adherence to the MedDiet using predefined dietary scores. Two main definitions of MedDiet used in the included studies referred to scores by Trichopoulou [12] and Fung [26]. Fewer reports adopted scores by Sofi [150] and Buckland [55]. Differences between scores concerned mostly cut-off points for moderate alcohol consumption and a way of handling healthy fat intake. Five added studies derived MedDiet scores from principal component analysis [113, 114, 116, 122, 125].
Corresponding risk estimates were based on comparison of extreme quantiles (top quintile/quartile/tertile versus bottom) [17, 18, 113,114,115,116, 122, 124,125,126, 128,129,130,131,132,133,134,135,136,137,138,139,140], fixed cut-off points [20, 21, 112, 117,118,119,120, 123, 141,142,143,144,145,146,147,148,149], per standard deviation [121], per-tertile [127] or per-20 percentile increase in the MedDiet score [19]. Majority of studies used MedDiet scores evaluated in the baseline, whereas one study reported risk in the context of a 12-year change of adherence to the dietary pattern [19].
Main outcomes
According to the different clinical outcomes, risk of cancer mortality was evaluated in 18 cohort studies and one RCT (n = 71,145 cases); breast cancer risk in 12 cohort, one RCT (n = 35,373 incident cases) and 11 case–control studies (n = 10,615 prevalent cases); colorectal cancer risk in nine cohort, one case–cohort (n = 26,185 incident cases) and seven case–control studies (n = 9683 prevalent cases); prostate cancer risk in five cohort, one case–cohort (n = 36,006 incident cases) and five case–control studies (n = 2466 prevalent cases); respiratory cancer risk in four cohort and one case–cohort studies (n = 12,730 incident cases); gastric cancer risk in three cohort, one case–cohort (n = 2343 incident cases) and three case–control studies (n = 1517 prevalent cases); liver cancer risk in three cohort (n = 1274 incident cases) and one case–control study (n = 518 prevalent cases); bladder in three cohort (n = 5844 incident cases) and one case–control study (n = 690 prevalent cases); pancreatic cancer risk in two cohort, one case–cohort (n = 1436 incident cases) and one case–control study (n = 688 prevalent cases); blood cancer risk in two cohort (n = 3614 incident cases) and two case–control studies (n = 691 prevalent cases); esophageal cancer in one cohort, one case–cohort (n = 1181 incident cases) and one case–control study (n = 304 prevalent cases); head and neck in one cohort (n = 1868 incident cases) and eight case–control studies (n = 4601 prevalent cases); endometrial cancer in one cohort (n = 1392 incident cases) and three case–control studies (n = 2355 prevalent cases); biliary tract (n = 163 incident cases), gallbladder (n = 77 incident cases), ovarian (n = 696 incident cases), skin cancer (n = 1436 incident cases) and glioma risk (n = 2313 incident cases) in one cohort study, respectively. Among cancer survivors, eight cohort studies summarized all-cause mortality (n = 4883 cases), cancer-specific mortality in four cohort studies (n = 1790 cases), and cancer reoccurrence in one cohort study (n = 92 cases).
Pooled estimates from random-effects models are summarized in Table 2 and corresponding forest plots are presented in ESM Figs. 2–22. Highest versus lowest adherence to the MedDiet was associated with a lower risk of cancer mortality in cohort studies (RRcohort: 0.87, 95% CI 0.82–0.92; I2 = 83%), but not in one RCT (RRRCT: 0.75, 95% CI 0.17–3.33, I2 = NA). Among cancer survivors, there was no association between the adherence to the MedDiet and cancer mortality risk (RRcohort: 0.96, 95% CI 0.82–1.11; I2 = 0%); however, an inverse association was observed in relation to all-cause mortality (RRcohort: 0.75, 95% CI 0.66–0.86, I2 = 41%). An inverse association of breast cancer with highest adherence to the MedDiet was found in one RCT (RRRCT: 0.41, 95% CI 0.19–0.87, I2 = NA) and observational studies (RRobservational: 0.94, 95% CI 0.90–0.97, I2 = 31%). However, considering separate designs there was a risk reduction in case–control studies (ORcase–control: 0.87, 95% CI 0.82–0.93, I2 = 6%), but not in cohort studies (RRcohort: 0.97, 95% CI 0.94–1.00, I2 = 0%). Regarding colorectal cancer, the highest adherence to the MedDiet was linked to a reduced risk (RRobservational: 0.83, 95% CI 0.76–0.90, I2 = 82%; ORcase–control: 0.64, 95% CI 0.52–0.79, I2 = 89%, RRcohort: 0.92, 95% CI 0.87–0.99, I2 = 50%). Furthermore, inverse associations between adherence to the MedDiet and risk of head and neck (RRobservational: 0.56, 95% CI 0.44–0.72; I2 = 91%, ORcase–control: 0.54, 95% CI 0.40–0.72, I2 = 92%; RRcohort: 0.73, 95% CI 0.60–0.89, I2 = NA), bladder (RRobservational: 0.87, 95% CI 0.76–0.98; I2 = 38%, ORcase–control: 0.66, 95% CI 0.47–0.93, I2 = NA; RRcohort: 0.89, 95% CI 0.81–0.97, I2 = 11%), gastric (RRobservational: 0.70, 95% CI 0.61–0.80; I2 = 52%, ORcase–control: 0.63 95% CI 0.53–0.75, I2 = 34%; RRcohort: 0.77, 95% CI 0.64–0.92, I2 = 44%), liver (RRobservational: 0.64, 95% CI 0.54–0.75, I2 = 0%; ORcase–control: 0.51, 95% CI 0.34–0.77, I2 = NA; RRcohort: 0.67, 95% CI 0.56–0.80, I2 = 0%), and respiratory (RRcohort: 0.84, 95% CI 0.76–0.94; I2 = 42%) cancers were found, respectively. Consistently no effect of adhering to the MedDiet was observed in relation to blood (RRobservational: 0.94, 95% CI 0.88–1.02, I2 = 0%; ORcase–control: 0.89, 95% CI 0.68–1.18, I2 = 0%; RRcohort 0.95, 95% CI 0.88–1.02, I2 = 0%) and prostate cancer (RRobservational: 0.98, 95% CI 0.93–1.04, I2 = 39%; ORcase–control: 0.76, 95% CI 0.52–1.13, I2 = 6%; RRcohort 0.98, 95% CI 0.94–1.02, I2 = 28%). Additionally no associations were observed for endometrial, esophageal and pancreatic cancer in observational studies (RRobservational: 0.67, 95% CI 0.41–1.11, I2 = 91%; RRobservational: 0.64, 95% CI 0.35–1.16, I2 = 81%; RRobservational: 0.80, 95% CI 0.60–1.06, I2 = 79%, respectively) with contrary findings from cohort (RRcohort: 0.98, 95% CI 0.82–1.17, I2 = NA; RRcohort: 0.85, 95% CI 0.67–1.09, I2 = 0%; RRcohort: 0.92, 95% CI 0.81–1.05, I2 = 0%, respectively) and case–control studies (ORcase–control: 0.58, 95% CI 0.35–0.95, I2 = 77%; ORcase–control: 0.26, 95% CI 0.13–0.52, I2 = NA; ORcase–control: 0.48, 95% CI 0.35–0.66, I2 = NA, respectively).
Subgroup analysis
None of the effect estimates was modified by the type of MedDiet score or geographical localization of study. Both menopausal status neither receptor expression pattern did not change the effect estimate for breast cancer. By specifying anatomical location of colorectal cancer, the general inverse association was re-established for distal colon and rectum (RR: 0.88, 95% CI 0.79 to 0.96, I2 = 0% and RR: 0.86, 95% CI 0.75–0.98, I2 = 42%, respectively), but not for proximal colon (RR: 1.01, 95% CI 0.93–1.09, I2 = 0%). The corresponding effect estimates are summarized in ESM Tables 2–4.
Components of the MedDiet and risk of cancer
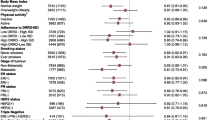
Summary risk ratios for the components of the MedDiet score are presented in Fig. 1. We found an inverse association between alcohol consumption within the recommended range compared to higher consumption (RR: 0.92, 95% CI 0.87–0.97), whole grain intake (RR: 0.93, 95% CI 0.88–0.98), fruit intake (RR: 0.94, 95% CI 0.91–0.97) as well as vegetable intake (RR: 0.96, 95% CI 0.94–0.98) and overall cancer risk. No associations were identified for cereals, dairy, fish, legumes, meat, nuts, and olive oil.
Publication bias
The results of Egger’s linear regression test did not support the presence of publication bias for cancer mortality (P = 0.55), breast cancer (P = 0.94), and colorectal cancer (P = 0.74) following comparison between highest and lowest adherence to the MedDiet. Funnel plots were created for analyses including at least 10 studies. Visual inspection of the plots suggested low asymmetry for colorectal cancer, as well as moderate asymmetry for cancer mortality and breast cancer, implying that publication bias might be affecting these associations (ESM Figs. 23–25).
Certainty of evidence
Application of the NutriGrade tool to the results from cohort studies resulted in moderate certainty of evidence for cancer mortality and colorectal cancer risk. Low certainty of evidence was found for incidence of bladder, blood, breast, gastric, liver, prostate and respiratory cancer as well as all-cause mortality among survivors. In RCTs certainty of evidence for breast cancer and cancer mortality was low. The credibility of findings for remaining site-specific cancers, cancer mortality and reoccurrence among cancer survivors was rated as very low, suggesting very low confidence in effect estimates (Table 2, ESM Table 5).
Discussion
In this updated systematic review, we meta-analysed current evidence on the association between adherence to MedDiet pattern and the risk of cancer. We identified 44 new studies, which provided data for an additional one million participants. The present analysis confirmed our previous findings on the inverse association of adherence to MedDiet on cancer mortality and colorectal cancer risk [16]. Contrary to our earlier reports, we observed conflicting findings between case–control and cohort studies for breast cancer [16]. Lack of association in cohort studies might suggest that significant findings found in case–control studies could be explained by bias linked to study design. Therefore, we cannot conclude on presence of inverse association between MedDiet and breast cancer risk. Our finding corresponds with statement from Continuous Updated Project by the WCRF suggesting too limited evidence to draw a conclusion on the relationship of healthy dietary patterns and breast cancer [10]. For the first time, we were able to observe an inverse association between adherence to the MedDiet and bladder, gastric and lung cancer incidence, as well as all-cause mortality among cancer survivors. Moreover, the present report included new cancer subtypes such as skin cancer and glioma, as well as identified new studies for those comparisons represented previously by a single study. The certainty of the evidence, evaluated for the first time in these series of reviews, was judged as “moderate” for cancer mortality and colorectal cancer and “very low” to “low” for other cancer subtypes. The NutriGrade scoring system did consider only meta-analyses of RCTs and cohort studies [23], but not case–control studies. Similarly, the evidence which was the basis for the 3rd WCRF report, considered only RCTs, cohort studies, and nested case–control studies. Individual case–control studies were not anymore considered due to limitation such as recall bias [10]. Considering the fact that in the current update we were able to identify only two RCTs with a very limited sample size, a major focus when interpreting our results should be put on findings from cohorts with a supportive role of case–control studies.
In 2014, Fardet and Rock referred to analyses of single nutrients or food groups as a reductionist approach not adequate in studies on the preventive effects of nutrition in chronic diseases such as cancer [151]. In contrast, dietary patterns may take into account synergistic and antagonistic interactions between the components of a food matrix, thus yielding a holistic net effect of diet [9]. Adherence to high-quality diets, such as the Healthy Eating Index (HEI) or the alternate HEI was inversely associated with cancer risk by approximately 15% [152]. In our analyses, the beneficial associations of the complete MedDiet on cancer was only reflected by inverse associations between overall cancer risk and fruit, vegetables, whole grains, and moderate alcohol intake, but not for fish, dairy and nuts (Fig. 1). Nevertheless, these observations may provide some insights to explain the mechanisms of action of MedDiet components/bioactive substances [153].
Fruits, vegetables, legumes and whole-grain products are a rich source of dietary fibre. Strong evidence from observational studies suggests a protective role of fibre mostly against colorectal cancer [10]. However, higher intake of dietary fibre was linked to a reduced risk of several other types of neoplasms including breast, gastric and lung cancer as well [154,155,156]. Gut microbiota reduces digested fibre to short-chain fatty acids such as butyrate, which helps to maintain proper function of the intestinal epithelium, as well as to reduce oncogenic potential by inducing cell apoptosis [157]. Some experimental studies demonstrated a direct interaction between fibre and pattern recognition receptors modulating immune anti-tumour response [158]. By increasing stool bulk, fibre can also dilute and slow absorption of potential carcinogens [158]. In addition, fruits and vegetables provide a variety of phytochemicals with potential anti-cancer effects. Bioactive substances such as carotenoids, flavonoids, stilbenes, coumarins and tannins can act synergically to increase antioxidative capacity and reduce cell oxidative damage [159, 160]. Furthermore, these compounds were shown to inhibit signal-transducing pathways, cell proliferation and oncogene expression, as well as to induce cell-cycle arrest [161]. A meta-analysis of prospective observational studies yielded an inverse association between antioxidative phytochemicals intake or their plasma/serum levels and risk of cancer [162]. Whole grains contain alk(en)ylresorcinols, benzoxanizoids and phytosteroids, which exerted an inhibitory effect on model human cancer cells [163]. Frequent consumption of whole grains was observed to lower risk of cancer mortality and incidence [164,165,166].
Apart from providing protective compounds, adherence to the MedDiet pattern decreases exposure to potential carcinogens by omitting intake of detrimental food items. Thus, extensive consumption of red and processed meat are associated with an increased risk of cancer, especially colorectal [165, 167]. Both food groups are a potential source of N-nitroso compounds, polycyclic aromatic hydrocarbons, and heterocyclic amines known to be cancerogenic [168,169,170]. A recent meta-analysis suggested that the above-mentioned chemicals are associated both with increased risks of colorectal and gastric cancers [171, 172].
Alcohol predominately in the form of red wine represents the most controversial ´food group´ within the context of the associations of the MedDiet on cancer. Increased ROS synthesis, suppressed anti-tumour immune response as well as metabolization of ethanol into DNA-damaging acetaldehyde may all explain the positive association between alcohol intake and cancer [125, 126]. The 3rd WCRF report indicated that there is a “convincing” grade of evidence for a positive association between alcohol intake and risk of upper aerodigestive, breast, colorectal or liver cancers, irrespective of the type of drink [10]. Definitions of moderate alcohol intake differ between the various MedDiet scores. According to Trichopoulou et al., consumption of up to 50 g/days for men and 25 g/days for women in form of red wine is considered as moderate [12], whereas Fung et al. [92] set cut-off points at 25 g/days and 15 g/days, respectively. Potential anti-tumorigenic effects of red wine are attributed to its polyphenolic content, especially resveratrol [173]. Although our results suggested a small reduction in overall cancer risk for alcohol intake within the range, compared to higher alcohol consumption, the benefit from light-to-moderate consumption of wine on cancer risk in observational studies is inconclusive [174,175,176]. Risk estimates for several cancers based on MedDiet scores including alcohol did not differ from those simply adjusted for total alcohol intake [122, 145,146,147,148,149]. Consumption of wine together with meals is a part of the cultural heritage in Mediterranean countries, but it is less common in other countries [177]. Therefore, the promotion of wine drinking in countries, where it is not a habit seems pointless, as small benefits do not exclude potential harm.
As already stated in the previous versions of this systematic review, a major limitation of our findings is the inconsistency of the definition of the MedDiet pattern [16]. Initially, the phrase was coined on the basis of observation made in several communities in the Mediterranean basin in the 1960s. Dietary intake has changed significantly since that time, which was stated in follow-up reports from the Seven Countries Study [178]. Therefore, MedDiet should rather be considered as a set of local variants based on cultural setting, food price and availability [177]. Consequently, dietary indices adopted in nutritional epidemiology as a means to quantify adherence to the MedDiet show substantial differences both in the composition of score as well as cut-off points for specific components. A recent umbrella review identified 74 different MedDiet scores used among studies eligible for systematic reviews and meta-analyses [13]. Popular definitions such as traditional MedDiet or alternate MedDiet indices use cut-off points based on median intake in studied populations [12, 26], which may substantially differ between studied populations. For example, median intake of vegetables for men in the Italian subcohort of the EPIC-InterAct study was 291 g/days, whereas the respective value in the Swedish subcohort was only 123 g/days [179]. A potential tool to address dissimilarities between MedDiet scores is the adoption of country-specific food environments [177]. For instance, olive oil, especially EVOO is rarely consumed in the US and northern Europe; therefore, MUFA-to-SFA ratio represents a more suitable measure of healthy fat intake [177]. However, little is known whether the use of these correction factors may result in equivalent preventive effects of MedDiet against cancer. Future studies need to focus on the use of literature-cased cut-off points for food groups as well as on the question whether different adaptations of MedDiet will yield comparable health-related outcomes.
Another limitation is the fact that pooled estimates presented in this review are based predominately on cohort studies set in Europe and the US, whereas single reports covered data from Asia. Uneven distribution of geographical locations might contribute to increased heterogeneity of data due to differences in cancer prevalence, genetic factors, or the burden of environmental risks, which can modify the effect of diet. However, stratifying analyses for study location did not affect the identified risk estimates in the present study.
Our results are based on observational rather than experimental data, which limits the interpretation of our findings with respect to causality. The use of randomized controlled trials in nutrition is limited by the inability to maintain high compliance during the long term of follow-up. Therefore, the use of data from prospective cohort studies is reasonable. To increase trust in our estimates, we did not consider case–control studies, which are prone to recall bias.
Most of the included studies constructed risk models using scores derived from food frequency data assessed during recruitment to study. Diet quality can substantially change during a long follow-up period. Thus, baseline adherence to the dietary pattern does not have to represent the true exposure. A particular strength of our systematic review is the large number of included studies as well as corresponding cancer cases. Another advantage of our analysis was the use of the NutriGrade tool. While assessing the certainty of evidence is key to construct evidence-based recommendations, it is rarely adopted in meta-analyses in nutrition research. To our best knowledge, this systematic review represents the first summary of trustworthiness of associations between adherence to the MedDiet and risk of cancer.
Conclusion
In conclusion, this systematic review and meta-analysis provides an updated body of evidence on the association between adherence to the MedDiet and risk of cancer. Our results suggest that highest adherence to the MedDiet was inversely associated with risk of cancer mortality in the general population, and all-cause mortality among cancer survivors as well as colorectal, head and neck, respiratory, gastric, liver and bladder cancer risks. However, the very low to moderate certainty of evidence found in this update requires a conservative interpretation of our findings.
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941–1953. https://doi.org/10.1002/ijc.31937
Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez-Jaramillo P, Gupta R, Diaz R, Avezum A, Oliveira GBF, Wielgosz A, Parambath SR, Mony P, Alhabib KF, Temizhan A, Ismail N, Chifamba J, Yeates K, Khatib R, Rahman O, Zatonska K, Kazmi K, Wei L, Zhu J, Rosengren A, Vijayakumar K, Kaur M, Mohan V, Yusufali A, Kelishadi R, Teo KK, Joseph P, Yusuf S (2019) Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. https://doi.org/10.1016/S0140-6736(19)32007-0
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2018) Global Cancer Observatory: Cancer Tomorrow. International Agency for Research on Cancer. https://gco.iarc.fr/tomorrow. Accessed 24 Nov 2019
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL (2019) Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clin 69(5):363–385. https://doi.org/10.3322/caac.21565
Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A (2018) Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 68(1):31–54. https://doi.org/10.3322/caac.21440
Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, Deas A, Elliss-Brookes L, Gavin A, Hounsome L, Huws D, Ormiston-Smith N, Shelton J, White C, Parkin DM (2018) The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer 118(8):1130–1141. https://doi.org/10.1038/s41416-018-0029-6
Parkin DM, Boyd L, Walker LC (2011) The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 105(Suppl 2):S77–81. https://doi.org/10.1038/bjc.2011.489
Zhang FF, Cudhea F, Shan Z, Michaud DS, Imamura F, Eom H, Ruan M, Rehm CD, Liu J, Du M, Kim D, Lizewski L, Wilde P, Mozaffarian D (2019) Preventable Cancer Burden Associated With Poor Diet in the United States. JNCI Cancer Spectr 3(2):pkz34. https://doi.org/10.1093/jncics/pkz034
Tapsell LC, Neale EP, Satija A, Hu FB (2016) Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr 7(3):445–454. https://doi.org/10.3945/an.115.011718
World Cancer Research Fund/American Institute for Cancer Research (2018) Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report. http://www.dietandcancerreport.org/. Accessed 24 Nov 2019
Menotti A, Puddu PE (2015) How the seven countries study contributed to the definition and development of the mediterranean diet concept: a 50-year journey. Nutri Metab Cardiovas Dis 25(3):245–252. https://doi.org/10.1016/j.numecd.2014.12.001
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D (2003) Adherence to a mediterranean diet and survival in a Greek population. New Engl J Med 348(26):2599–2608. https://doi.org/10.1056/NEJMoa025039
Galbete C, Schwingshackl L, Schwedhelm C, Boeing H, Schulze MB (2018) Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur J Epidemiol 33(10):909–931. https://doi.org/10.1007/s10654-018-0427-3
Schwingshackl L, Hoffmann G (2014) Adherence to mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer 135(8):1884–1897. https://doi.org/10.1002/ijc.28824
Schwingshackl L, Hoffmann G (2015) Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med 4(12):1933–1947. https://doi.org/10.1002/cam4.539
Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G (2017) Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu9101063
Haridass V, Ziogas A, Neuhausen SL, Anton-Culver H, Odegaard AO (2018) Diet quality scores inversely associated with postmenopausal breast cancer risk are not associated with premenopausal breast cancer risk in the california teachers study. J Nutr 148(11):1830–1837. https://doi.org/10.1093/jn/nxy187
Hashemian M, Farvid MS, Poustchi H, Murphy G, Etemadi A, Hekmatdoost A, Kamangar F, Sheikh M, Pourshams A, Sepanlou SG, Fazeltabar Malekshah A, Khoshnia M, Gharavi A, Brennan PJ, Boffetta P, Dawsey SM, Reedy J, Subar AF, Abnet CC, Malekzadeh R (2019) The application of six dietary scores to a Middle Eastern population: a comparative analysis of mortality in a prospective study. Eur J Epidemiol 34(4):371–382. https://doi.org/10.1007/s10654-019-00508-3
Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB (2017) Association of changes in diet quality with total and cause-specific mortality. N Engl J Med 377(2):143–153. https://doi.org/10.1056/NEJMoa1613502
Kuan AS, Green J, Kitahara CM, Berrington de Gonzalez A, Key T, Reeves G, Floud S, Balkwill A, Bradbury K, Liao LM, Freedman ND, Beral V, Sweetland S (2019) Diet and risk of glioma: combined analysis of three large prospective studies in the UK and USA. Neurooncology. https://doi.org/10.1093/neuonc/noz013
Mahamat-Saleh Y, Cervenka I, Al Rahmoun M, Savoye I, Mancini FR, Trichopoulou A, Boutron-Ruault MC, Kvaskoff M (2019) Mediterranean dietary pattern and skin cancer risk: a prospective cohort study in French women. Am J Clin Nutr 110(4):993–1002. https://doi.org/10.1093/ajcn/nqz173
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Schwingshackl L, Knuppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopantelis E, Iqbal K, Aleksandrova K, Lorkowski S, Leitzmann MF, Kroke A, Boeing H (2016) Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 7(6):994–1004. https://doi.org/10.3945/an.116.013052
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 82(1):163–173. https://doi.org/10.1093/ajcn.82.1.163
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019) Cochrane handbook for systematic reviews of interventions 2nd, Edition edn. Wiley, Chichester
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. J Stat Softw 1(3):2010. https://doi.org/10.18637/jss.v036.i03
Cuenca-Garcia M, Artero EG, Sui X, Lee DC, Hebert JR, Blair SN (2014) Dietary indices, cardiovascular risk factors and mortality in middle-aged adults: findings from the Aerobics Center Longitudinal Study. Ann Epidemiol 24(4):297–303.e292. https://doi.org/10.1016/j.annepidem.2014.01.007
George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML (2014) Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the women's health initiative observational study: evidence to inform national dietary guidance. Am J Epidemiol 180(6):616–625. https://doi.org/10.1093/aje/kwu173
Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN (2015) Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr 101(3):587–597. https://doi.org/10.3945/ajcn.114.090688
Knoops KTB, de Groot L, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA (2004) Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women—The HALE project. JAMA 292(12):1433–1439. https://doi.org/10.1001/jama.292.12.1433
Lagiou P, Trichopoulos D, Sandin S, Lagiou A, Mucci L, Wolk A, Weiderpass E, Adami H-O (2006) Mediterranean dietary pattern and mortality among young women: a cohort study in Sweden. Br J Nutr 96(2):384–392. https://doi.org/10.1079/bjn20061824
Lassale C, Gunter MJ, Romaguera D, Peelen LM, Van der Schouw YT, Beulens JWJ, Freisling H, Muller DC, Ferrari P, Huybrechts I, Fagherazzi G, Boutron-Ruault M-C, Affret A, Overvad K, Dahm CC, Olsen A, Roswall N, Tsilidis KK, Katzke VA, Kuehn T, Buijsse B, Quiros J-R, Sanchez-Cantalejo E, Etxezarreta N, Maria Huerta J, Barricarte A, Bonet C, Khaw K-T, Key TJ, Trichopoulou A, Bamia C, Lagiou P, Palli D, Agnoli C, Tumino R, Fasanelli F, Panico S, Bueno-de-Mesquita HB, Boer JMA, Sonestedt E, Nilsson LM, Renstrom F, Weiderpass E, Skeie G, Lund E, Moons KGM, Riboli E, Tzoulaki I (2016) Diet Quality Scores and Prediction of All-Cause, Cardiovascular and Cancer Mortality in a Pan-European Cohort Study. Plos One. https://doi.org/10.1371/journal.pone.0159025
Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, Fung TT, Li S, Willett WC, Rimm EB, Hu FB (2014) The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr 99(1):172–180. https://doi.org/10.3945/ajcn.113.068106
Martinez-Gonzalez MA, Guillen-Grima F, De Irala J, Ruiz-Canela M, Bes-Rastrollo M, Beunza JJ, Lopez del Burgo C, Toledo E, Carlos S, Sanchez-Villegas A (2012) The Mediterranean diet is associated with a reduction in premature mortality among middle-aged adults. J Nutr 142(9):1672–1678. https://doi.org/10.3945/jn.112.162891
Menotti A, Alberti-Fidanza A, Fidanza F, Lanti M, Fruttini D (2012) Factor analysis in the identification of dietary patterns and their predictive role in morbid and fatal events. Pub Health Nutr 15(7):1232–1239. https://doi.org/10.1017/s1368980011003235
Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF (2014) Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 144(6):881–889. https://doi.org/10.3945/jn.113.189407
Tognon G, Nilsson LM, Lissner L, Johansson I, Hallmans G, Lindahl B, Winkvist A (2012) The Mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. J Nutr 142(8):1547–1553. https://doi.org/10.3945/jn.112.160499
Vormund K, Braun J, Rohrmann S, Bopp M, Ballmer P, Faeh D (2015) Mediterranean diet and mortality in Switzerland: an alpine paradox? Eur J Nutr 54(1):139–148. https://doi.org/10.1007/s00394-014-0695-y
Whalen KA, Judd S, McCullough ML, Flanders WD, Hartman TJ, Bostick RM (2017) Paleolithic and Mediterranean diet pattern scores are inversely associated with all-cause and cause-specific mortality in adults. J Nutr 147(4):612–620. https://doi.org/10.3945/jn.116.241919
Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, Dahm CC, Overvad K, Olsen A, Tjonneland A, Cottet V, Boutron-Ruault M-C, Morois S, Grote V, Teucher B, Boeing H, Buijsse B, Trichopoulos D, Adarakis G, Tumino R, Naccarati A, Panico S, Palli D, Bueno-de-Mesquita HB, van Duijnhoven FJB, Peeters PHM, Engeset D, Skeie G, Lund E, Sanchez M-J, Barricarte A, Huerta J-M, Ramon Quiros J, Dorronsoro M, Ljuslinder I, Palmqvist R, Drake I, Key TJ, Khaw K-T, Wareham N, Romieu I, Fedirko V, Jenab M, Romaguera D, Norat T, Trichopoulou A (2013) Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol 28(4):317–328. https://doi.org/10.1007/s10654-013-9795-x
Jones P, Cade JE, Evans CE, Hancock N, Greenwood DC (2017) The Mediterranean diet and risk of colorectal cancer in the UK Women’s Cohort Study. Int J Epidemiol 46(6):1786–1796. https://doi.org/10.1093/ije/dyx155%JInternationalJournalofEpidemiology
Park S-Y, Boushey CJ, Wilkens LR, Haiman CA, Le Marchand L (2017) High-quality diets associate with reduced risk of colorectal cancer: analyses of diet quality indexes in the multiethnic cohort. Gastroenterology 153(2):386. https://doi.org/10.1053/j.gastro.2017.04.004
Reedy J, Mitrou PN, Krebs-Smith SM, Wirfalt E, Flood A, Kipnis V, Leitzmann M, Mouw T, Hollenbeck A, Schatzkin A, Subar AF (2008) Index-based dietary patterns and risk of colorectal cancer. Am J Epidemiol 168(1):38–48. https://doi.org/10.1093/aje/kwn097
Vargas AJ, Neuhouser ML, George SM, Thomson CA, Ho GYF, Rohan TE, Kato I, Nassir R, Hou L, Manson JE (2016) Diet quality and colorectal cancer risk in the women's health initiative observational study. Am J Epidemiol 184(1):23–32. https://doi.org/10.1093/aje/kwv304
Buckland G, Travier N, Cottet V, Gonzalez CA, Lujan-Barroso L, Agudo A, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, May A, Bueno-de-Mesquita HB, Duijnhoven FJB, Key TJ, Allen N, Khaw KT, Wareham N, Romieu I, McCormack V, Boutron-Ruault M, Clavel-Chapelon F, Panico S, Agnoli C, Palli D, Tumino R, Vineis P, Amiano P, Barricarte A, Rodriguez L, Sanchez MJ, Chirlaque MD, Kaaks R, Teucher B, Boeing H, Bergmann MM, Overvad K, Dahm CC, Tjonneland A, Olsen A, Manjer J, Wirfalt E, Hallmans G, Johansson I, Lund E, Hjartaker A, Skeie G, Vergnaud AC, Norat T, Romaguera D, Riboli E (2013) Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer 132(12):2918–2927. https://doi.org/10.1002/ijc.27958
Cade JE, Taylor EF, Burley VJ, Greenwood DC (2011) Does the Mediterranean dietary pattern or the healthy diet index influence the risk of breast cancer in a large british cohort of women? Eur J Clin Nutr 65(8):920–928. https://doi.org/10.1038/ejcn.2011.69
Couto E, Sandin S, Lof M, Ursin G, Adami H-O, Weiderpass E (2013) Mediterranean dietary pattern and risk of breast cancer. PLoS One. https://doi.org/10.1371/journal.pone.0055374
Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD (2006) Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr 136(2):466–472
van den Brandt PA, Schulpen M (2017) Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer 140(10):2220–2231. https://doi.org/10.1002/ijc.30654
Ax E, Garmo H, Grundmark B, Bill-Axelson A, Holmberg L, Becker W, Zethelius B, Cederholm T, Sjogren P (2014) Dietary patterns and prostate cancer risk: report from the population based ULSAM cohort study of swedish men. Nutr Cancer Int J 66(1):77–87. https://doi.org/10.1080/01635581.2014.851712
Bosire C, Stampfer MJ, Subar AF, Park Y, Kirkpatrick SI, Chiuve SE, Hollenbeck AR, Reedy J (2013) Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. Am J Epidemiol 177(6):504–513. https://doi.org/10.1093/aje/kws261
Kenfield SA, Dupre N, Richman EL, Stampfer MJ, Chan JM, Giovannucci EL (2014) Mediterranean diet and prostate cancer risk and mortality in the health professionals follow-up study. Eur Urol 65(5):887–894. https://doi.org/10.1016/j.eururo.2013.08.009
Buckland G, Agudo A, Lujan L, Jakszyn P, Bueno-de-Mesquita HB, Palli D, Boeing H, Carneiro F, Krogh V, Sacerdote C, Tumino R, Panico S, Nesi G, Manjer J, Regner S, Johansson I, Stenling R, Sanchez M-J, Dorronsoro M, Barricarte A, Navarro C, Ramon Quiros J, Allen NE, Key TJ, Bingham S, Kaaks R, Overvad K, Jensen M, Olsen A, Tjonneland A, Peeters PHM, Numans ME, Ocke MC, Clavel-Chapelon F, Morois S, Boutron-Ruault M-C, Trichopoulou A, Lagiou P, Trichopoulos D, Lund E, Couto E, Boffeta P, Jenab M, Riboli E, Romaguera D, Mouw T, Gonzalez CA (2010) Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr 91(2):381–390. https://doi.org/10.3945/ajcn.2009.28209
Li W-Q, Park Y, Wu JW, Ren J-S, Goldstein AM, Taylor PR, Hollenbeck AR, Freedman ND, Abnet CC (2013) Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol 11(9):1130–U1136. https://doi.org/10.1016/j.cgh.2013.03.023
Li W-Q, Park Y, McGlynn KA, Hollenbeck AR, Taylor PR, Goldstein AM, Freedman ND (2014) Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology 60(2):588–597. https://doi.org/10.1002/hep.27160
Li W-Q, Park Y, Wu JW, Goldstein AM, Taylor PR, Hollenbeck AR, Freedman ND, Abnet CC (2014) Index-based dietary patterns and risk of head and neck cancer in a large prospective study. Am J Clin Nutr 99(3):559–566. https://doi.org/10.3945/ajcn.113.073163
George SM, Ballard R, Shikany JM, Crane TE, Neuhouser ML (2015) A prospective analysis of diet quality and endometrial cancer among 84,415 postmenopausal women in the Women's Health Initiative. Ann Epidemiol 25(10):788–793. https://doi.org/10.1016/j.annepidem.2015.05.009
Anic GM, Park Y, Subar AF, Schap TE, Reedy J (2016) Index-based dietary patterns and risk of lung cancer in the NIH-AARP diet and health study. Eur J Clin Nutr 70(1):123–129. https://doi.org/10.1038/ejcn.2015.122
Hodge AM, Bassett JK, Shivappa N, Hebert JR, English DR, Giles GG, Severi G (2016) Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control 27(7):907–917. https://doi.org/10.1007/s10552-016-0770-1
Maisonneuve P, Shivappa N, Hebert JR, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G, Gnagnarella P (2016) Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr 55(3):1069–1079. https://doi.org/10.1007/s00394-015-0920-3
Dugue P-A, Hodge AM, Brinkman MT, Bassett JK, Shivappa N, Hebert JR, Hopper JL, English DR, Milne RL, Giles GG (2016) Association between selected dietary scores and the risk of urothelial cell carcinoma: a prospective cohort study. Int J Cancer 139(6):1251–1260. https://doi.org/10.1002/ijc.30175
Buckland G, Ros MM, Roswall N, Bueno-de-Mesquita HB, Travier N, Tjonneland A, Kiemeney LA, Sacerdote C, Tumino R, Ljungberg B, Gram IT, Weiderpass E, Skeie G, Malm J, Ehrnstrom R, Chang-Claude J, Mattiello A, Agnoli C, Peeters PH, Boutron-Ruault MC, Fagherazzi G, Clavel-Chapelon F, Nilsson LM, Amiano P, Trichopoulou A, Oikonomou E, Tsiotas K, Sanchez MJ, Overvad K, Quiros JR, Chirlaque MD, Barricarte A, Key TJ, Allen NE, Khaw KT, Wareham N, Riboli E, Kaaks R, Boeing H, Palli D, Romieu I, Romaguera D, Gonzalez CA (2014) Adherence to the Mediterranean diet and risk of bladder cancer in the EPIC cohort study. Int J Cancer 134(10):2504–2511. https://doi.org/10.1002/ijc.28573
Molina-Montes E, Sanchez M-J, Buckland G, Bueno-de-Mesquita HB, Weiderpass E, Amiano P, Wark PA, Kuehn T, Katzke V, Maria Huerta J, Ardanaz E, Ramon Quiros J, Affret A, His M, Boutron-Ruault M-C, Peeters PH, Ye W, Sund M, Boeing H, Iqbal K, Ohlsson B, Sonestedt E, Tjonneland A, Petersen KEN, Travis RC, Skeie G, Agnoli C, Panico S, Palli D, Tumino R, Sacerdote C, Freisling H, Huybrechts I, Overvad K, Trichopoulou A, Bamia C, Vasilopoulou E, Wareham N, Khaw K-T, Cross AJ, Ward HA, Riboli E, Duell EJ (2017) Mediterranean diet and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Br J Cancer 116(6):811–820. https://doi.org/10.1038/bjc.2017.14
Larsson SC, Hakansson N, Wolk A (2017) Healthy dietary patterns and incidence of biliary tract and gallbladder cancer in a prospective study of women and men. Eur J Cancer 70:42–47. https://doi.org/10.1016/j.ejca.2016.10.012
Xie J, Poole EM, Terry KL, Fung TT, Rosner BA, Willett WC, Tworoger SS (2014) A prospective cohort study of dietary indices and incidence of epithelial ovarian cancer. J Ovar Res. https://doi.org/10.1186/s13048-014-0112-4
Kim EHJ, Willett WC, Fung T, Rosner B, Holmes MD (2011) Diet quality indices and postmenopausal breast cancer survival. Nutr Cancer Int J 63(3):381–388. https://doi.org/10.1080/01635581.2011.535963
Jacobs S, Harmon BE, Ollberding NJ, Wilkens LR, Monroe KR, Kolonel LN, Le Marchand L, Boushey CJ, Maskarinec G (2016) Among 4 diet quality indexes, only the alternate mediterranean diet score is associated with better colorectal cancer survival and only in african american women in the multiethnic cohort. J Nutr 146(9):1746–1755. https://doi.org/10.3945/jn.116.234237
Fung TT, Kashambwa R, Sato K, Chiuve SE, Fuchs CS, Wu K, Giovannucci E, Ogino S, Hu FB, Meyerhardt JA (2014) Post diagnosis diet quality and colorectal cancer survival in women. PLoS One. https://doi.org/10.1371/journal.pone.0115377
Cottet V, Bonithon-Kopp C, Kronborg O, Santos L, Andreatta R, Boutron-Ruault MC, Faivre J, European Cancer Prevention Org S (2005) Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prevent 14(1):21–29. https://doi.org/10.1097/00008469-200502000-00004
Butler LM, Wu AH, Wang R, Koh WP, Yuan JM, Yu MC (2010) A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr 91(4):1013–1019. https://doi.org/10.3945/ajcn.2009.28572
Buckland G, Agudo A, Travier N, Huerta JM, Cirera L, Tormo MJ, Navarro C, Chirlaque MD, Moreno-Iribas C, Ardanaz E, Barricarte A, Etxeberria J, Marin P, Quiros JR, Redondo ML, Larranaga N, Amiano P, Dorronsoro M, Arriola L, Basterretxea M, Sanchez MJ, Molina E, Gonzalez CA (2011) Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br J Nutr 106(10):1581–1591. https://doi.org/10.1017/s0007114511002078
Mitrou PN, Kipnis V, Thiebaut ACM, Reedy J, Subar AF, Wirfalt E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, Schatzkin A (2007) Mediterranean dietary pattern and prediction of all-cause mortality in a US population—results from the NIH-AARP diet and health study. Arch Intern Med 167(22):2461–2468. https://doi.org/10.1001/archinte.167.22.2461
Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E (2010) The Mediterranean and dietary approaches to stop hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 92(6):1429–1435. https://doi.org/10.3945/ajcn.2010.29242
Couto E, Boffetta P, Lagiou P, Ferrari P, Buckland G, Overvad K, Dahm CC, Tjonneland A, Olsen A, Clavel-Chapelon F, Boutron-Ruault MC, Cottet V, Trichopoulos D, Naska A, Benetou V, Kaaks R, Rohrmann S, Boeing H, von Ruesten A, Panico S, Pala V, Vineis P, Palli D, Tumino R, May A, Peeters PH, Bueno-de-Mesquita HB, Buchner FL, Lund E, Skeie G, Engeset D, Gonzalez CA, Navarro C, Rodriguez L, Sanchez MJ, Amiano P, Barricarte A, Hallmans G, Johansson I, Manjer J, Wirfart E, Allen NE, Crowe F, Khaw KT, Wareham N, Moskal A, Slimani N, Jenab M, Romaguera D, Mouw T, Norat T, Riboli E, Trichopoulou A (2011) Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br J Cancer 104(9):1493–1499. https://doi.org/10.1038/bjc.2011.106
Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G (2013) Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann Oncol 24(10):2606–2611. https://doi.org/10.1093/annonc/mdt302
Haridass V (2015) Diet quality scores and risk of incident breast cancer in the California Teachers Study. Dissertation, University of California, Irvine. https://escholarship.org/uc/item/87t2942b. Accessed 24 Nov 2019
Hirko KA, Willett WC, Hankinson SE, Rosner BA, Beck AH, Tamimi RM, Eliassen AH (2016) Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat 155(3):579–588. https://doi.org/10.1007/s10549-016-3706-2
Bessaoud F, Tretarre B, Daures JP, Gerber M (2012) Identification of dietary patterns using two statistical approaches and their association with breast cancer risk: a case-control study in Southern France. Ann Epidemiol 22(7):499–510. https://doi.org/10.1016/j.annepidem.2012.04.006
Bosetti C, Gallus S, Trichopoulou A, Talamini R, Franceschi S, Negri E, La Vecchia C (2003) Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev 12(10):1091–1094
Bosetti C, Turati F, Dal Pont A, Ferraroni M, Polesel J, Negri E, Serraino D, Talamini R, La Vecchia C, Zeegers MP (2013) The role of Mediterranean diet on the risk of pancreatic cancer. Br J Cancer 109(5):1360–1366. https://doi.org/10.1038/bjc.2013.345
Demetriou CA, Hadjisavvas A, Loizidou MA, Loucaides G, Neophytou I, Sieri S, Kakouri E, Middleton N, Vineis P, Kyriacou K (2012) The mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: a case-control study. BMC Cancer 12:113. https://doi.org/10.1186/1471-2407-12-113
Dixon LB, Subar AF, Peters U, Weissfeld JL, Bresalier RS, Risch A, Schatzkin A, Hayes RB (2007) Adherence to the USDA food guide, DASH eating plan, and mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr 137(11):2443–2450. https://doi.org/10.1093/jn/137.11.2443
Kontou N, Psaltopoulou T, Soupos N, Polychronopoulos E, Xinopoulos D, Linos A, Panagiotakos DB (2012) Metabolic syndrome and colorectal cancer: the protective role of Mediterranean diet—a case-control study. Angiology 63(5):390–396. https://doi.org/10.1177/0003319711421164
Möller E, Galeone C, Andersson TML, Bellocco R, Adami H-O, Andrén O, Grönberg H, La Vecchia C, Mucci LA, Bälter K (2013) Mediterranean diet score and prostate cancer risk in a Swedish population-based case-control study. J Nutr Sci 2:e15–e15. https://doi.org/10.1017/jns.2013.2
Murtaugh MA, Sweeney C, Giuliano AR, Herrick JS, Hines L, Byers T, Baumgartner KB, Slattery ML (2008) Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the four-corners breast cancer study. Am J Clin Nutr 87(4):978–984. https://doi.org/10.1093/ajcn/87.4.978
Nkondjock A, Ghadirian P (2007) Diet quality and BRCA-associated breast cancer risk. Breast Cancer Res Treat 103(3):361–369. https://doi.org/10.1007/s10549-006-9371-0
Praud D, Bertuccio P, Bosetti C, Turati F, Ferraroni M, La Vecchia C (2014) Adherence to the Mediterranean diet and gastric cancer risk in Italy. Int J Cancer 134(12):2935–2941. https://doi.org/10.1002/ijc.28620
Samoli E, Lagiou A, Nikolopoulos E, Lagogiannis G, Barbouni A, Lefantzis D, Trichopoulos D, Brennan P, Lagiou P (2010) Mediterranean diet and upper aerodigestive tract cancer: the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study. Br J Nutr 104(9):1369–1374. https://doi.org/10.1017/s0007114510002205
Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC (2009) Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr 89(4):1145–1154. https://doi.org/10.3945/ajcn.2008.26915
Castello A, Pollan M, Buijsse B, Ruiz A, Casas AM, Baena-Canada JM, Lope V, Antolin S, Ramos M, Munoz M, Lluch A, de Juan-Ferre A, Jara C, Jimeno MA, Rosado P, Diaz E, Guillem V, Carrasco E, Perez-Gomez B, Vioque J, Boeing H, Martin M (2014) Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer 111(7):1454–1462. https://doi.org/10.1038/bjc.2014.434
Dalvi TB, Canchola AJ, Horn-Ross PL (2007) Dietary patterns, Mediterranean diet, and endometrial cancer risk. Cancer Causes Control 18(9):957–966. https://doi.org/10.1007/s10552-007-9037-1
Filomeno M, Bosetti C, Bidoli E, Levi F, Serraino D, Montella M, La Vecchia C, Tavani A (2015) Mediterranean diet and risk of endometrial cancer: a pooled analysis of three Italian case-control studies. Br J Cancer 112(11):1816–1821. https://doi.org/10.1038/bjc.2015.153
Filomeno M, Bosetti C, Garavello W, Levi F, Galeone C, Negri E, La Vecchia C (2014) The role of a Mediterranean diet on the risk of oral and pharyngeal cancer. Br J Cancer 111(5):981–986. https://doi.org/10.1038/bjc.2014.329
Grosso G, Biondi A, Galvano F, Mistretta A, Marventano S, Buscemi S, Drago F, Basile F (2014) Factors associated with colorectal cancer in the context of the Mediterranean diet: a case-control study. Nutr Cancer 66(4):558–565. https://doi.org/10.1080/01635581.2014.902975
Mourouti N, Kontogianni MD, Papavagelis C, Plytzanopoulou P, Vassilakou T, Malamos N, Linos A, Panagiotakos DB (2014) Adherence to the Mediterranean diet is associated with lower likelihood of breast cancer: a case-control study. Nutr Cancer 66(5):810–817. https://doi.org/10.1080/01635581.2014.916319
Pot GK, Stephen AM, Dahm CC, Key TJ, Cairns BJ, Burley VJ, Cade JE, Greenwood DC, Keogh RH, Bhaniani A, McTaggart A, Lentjes MA, Mishra G, Brunner EJ, Khaw KT (2014) Dietary patterns derived with multiple methods from food diaries and breast cancer risk in the UK Dietary Cohort Consortium. Eur J Clin Nutr 68(12):1353–1358. https://doi.org/10.1038/ejcn.2014.135
Turati F, Trichopoulos D, Polesel J, Bravi F, Rossi M, Talamini R, Franceschi S, Montella M, Trichopoulou A, La Vecchia C, Lagiou P (2014) Mediterranean diet and hepatocellular carcinoma. J Hepatol 60(3):606–611. https://doi.org/10.1016/j.jhep.2013.10.034
Whalen KA, McCullough M, Flanders WD, Hartman TJ, Judd S, Bostick RM (2014) Paleolithic and Mediterranean diet pattern scores and risk of incident, sporadic colorectal adenomas. Am J Epidemiol 180(11):1088–1097. https://doi.org/10.1093/aje/kwu235
Askari F, Beyzaei B, Tehrani A, Kardoust Parizi M, Nosratmirshekarlou E, Rashidkhan B (2016) Adherence to mediterranean-style dietary pattern and risk of prostate cancer: a case-control study in Iran. Pak J Nutr 15:305–311. https://doi.org/10.3923/pjn.2016.305.311
Campagna M, Cocco P, Zucca M, Angelucci E, Gabbas A, Latte GC, Uras A, Rais M, Sanna S, Ennas MG (2015) Risk of lymphoma subtypes and dietary habits in a Mediterranean area. Cancer Epidemiol 39(6):1093–1098. https://doi.org/10.1016/j.canep.2015.09.001
Castello A, Boldo E, Amiano P, Castano-Vinyals G, Aragones N, Gomez-Acebo I, Peiro R, Jimenez-Moleon JJ, Alguacil J, Tardon A, Cecchini L, Lope V, Dierssen-Sotos T, Mengual L, Kogevinas M, Pollan M, Perez-Gomez B (2018) Mediterranean dietary pattern is associated with low risk of aggressive prostate cancer: MCC-Spain Study. J Urol 199(2):430–437. https://doi.org/10.1016/j.juro.2017.08.087
Castello A, Boldo E, Perez-Gomez B, Lope V, Altzibar JM, Martin V, Castano-Vinyals G, Guevara M, Dierssen-Sotos T, Tardon A, Moreno V, Puig-Vives M, Llorens-Ivorra C, Alguacil J, Gomez-Acebo I, Castilla J, Gracia-Lavedan E, Davila-Batista V, Kogevinas M, Aragones N, Amiano P, Pollan M (2017) Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 103:8–15. https://doi.org/10.1016/j.maturitas.2017.06.020
Giraldi L, Panic N, Cadoni G, Boccia S, Leoncini E (2017) Association between Mediterranean diet and head and neck cancer: results of a large case-control study in Italy. Eur J Cancer Prev 26(5):418–423. https://doi.org/10.1097/cej.0000000000000277
Rosato V, Guercio V, Bosetti C, Negri E, Serraino D, Giacosa A, Montella M, La Vecchia C, Tavani A (2016) Mediterranean diet and colorectal cancer risk: a pooled analysis of three Italian case-control studies. Br J Cancer 115(7):862–865. https://doi.org/10.1038/bjc.2016.245
Stojanovic J, Giraldi L, Arzani D, Pastorino R, Biondi A, Persiani R, Boccia S, Leoncini E (2017) Adherence to Mediterranean diet and risk of gastric cancer: results of a case-control study in Italy. Eur J Cancer Prev 26(6):491–496. https://doi.org/10.1097/cej.0000000000000371
Turati F, Bravi F, Polesel J, Bosetti C, Negri E, Garavello W, Taborelli M, Serraino D, Libra M, Montella M, Decarli A, Ferraroni M, La Vecchia C (2017) Adherence to the Mediterranean diet and nasopharyngeal cancer risk in Italy. Cancer Causes Control 28(2):89–95. https://doi.org/10.1007/s10552-017-0850-x
Wang C, Lin XL, Fan YY, Liu YT, Zhang XL, Lu YK, Xu CH, Chen YM (2016) Diet quality scores and risk of nasopharyngeal carcinoma in chinese adults: a case-control study. Nutrients 8(3):112. https://doi.org/10.3390/nu8030112
de Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N (1998) Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med 158(11):1181–1187. https://doi.org/10.1001/archinte.158.11.1181
Toledo E, Salas-Salvado J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, Corella D, Fito M, Hu FB, Aros F, Gomez-Gracia E, Romaguera D, Ortega-Calvo M, Serra-Majem L, Pinto X, Schroder H, Basora J, Sorli JV, Bullo M, Serra-Mir M, Martinez-Gonzalez MA (2015) Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med 175(11):1752–1760. https://doi.org/10.1001/jamainternmed.2015.4838. Erratum in: JAMA Intern Med 2018:178(12):1731–1732
Bravi F, Spei ME, Polesel J, Di Maso M, Montella M, Ferraroni M, Serraino D, Libra M, Negri E, La Vecchia C, Turati F (2018) Mediterranean diet and bladder cancer risk in Italy. Nutrients. https://doi.org/10.3390/nu10081061
Castello A, Amiano P, Fernandez de Larrea N, Martin V, Alonso MH, Castano-Vinyals G, Perez-Gomez B, Olmedo-Requena R, Guevara M, Fernandez-Tardon G, Dierssen-Sotos T, Llorens-Ivorra C, Huerta JM, Capelo R, Fernandez-Villa T, Diez-Villanueva A, Urtiaga C, Castilla J, Jimenez-Moleon JJ, Moreno V, Davila-Batista V, Kogevinas M, Aragones N, Pollan M (2019) Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr 58(4):1495–1505. https://doi.org/10.1007/s00394-018-1674-5
Castello A, Fernandez de Larrea N, Martin V, Davila-Batista V, Boldo E, Guevara M, Moreno V, Castano-Vinyals G, Gomez-Acebo I, Fernandez-Tardon G, Peiro R, Olmedo-Requena R, Capelo R, Navarro C, Pacho-Valbuena S, Perez-Gomez B, Kogevinas M, Pollan M, Aragones N (2018) High adherence to the Western, Prudent, and Mediterranean dietary patterns and risk of gastric adenocarcinoma: MCC-Spain study. Gastric Cancer 21(3):372–382. https://doi.org/10.1007/s10120-017-0774-x
Jafari Nasab S, Bahrami A, Rafiee P, Hekmatdoust A, Ghanavati M, Rashidkhani B, Sadeghi A, Asadzadeh Aghdaei H, Naja F, Hejazi E (2019) Healthy eating index-2010 and mediterranean-style dietary pattern score and the risk of colorectal cancer and adenoma: a case-control study. Nutr Cancer. https://doi.org/10.1080/01635581.2019.1683212
Jalilpiran Y, Dianatinasab M, Zeighami S, Bahmanpour S, Ghiasvand R, Mohajeri SAR, Faghih S (2018) Western dietary pattern, but not mediterranean dietary pattern, increases the risk of prostate cancer. Nutr Cancer 70(6):851–859. https://doi.org/10.1080/01635581.2018.1490779
Krusinska B, Wadolowska L, Slowinska MA, Biernacki M, Drozdowski M, Chadzynski T (2018) Associations of dietary patterns and metabolic-hormone profiles with breast cancer risk: a case-control study. Nutrients. https://doi.org/10.3390/nu10122013
Ricceri F, Giraudo MT, Fasanelli F, Milanese D, Sciannameo V, Fiorini L, Sacerdote C (2017) Diet and endometrial cancer: a focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer 17(1):757. https://doi.org/10.1186/s12885-017-3754-y
Russo GI, Solinas T, Urzi D, Privitera S, Campisi D, Cocci A, Carini M, Madonia M, Cimino S, Morgia G (2019) Adherence to Mediterranean diet and prostate cancer risk in Sicily: population-based case-control study. Int J Impot Res 31(4):269–275. https://doi.org/10.1038/s41443-018-0088-5
Salvatore Benito A, Valero Zanuy MA, Alarza Cano M, Ruiz Alonso A, Alda Bravo I, Rogero Blanco E, Maiz Jimenez M, Leon Sanz M (2019) Adherence to Mediterranean diet: a comparison of patients with head and neck cancer and healthy population. Endocrinol Diab Nutr 66(7):417–424. https://doi.org/10.1016/j.endinu.2018.12.002
Saraiya V, Bradshaw P, Meyer K, Gammon M, Slade G, Brennan P, Abedi-Ardekani B, Olshan A (2020) The association between diet quality and cancer incidence of the head and neck. Cancer Causes Control 31(2):193–202. https://doi.org/10.1007/s10552-019-01261-4
Solans M, Castello A, Benavente Y, Marcos-Gragera R, Amiano P, Gracia-Lavedan E, Costas L, Robles C, Gonzalez-Barca E, de la Banda E, Alonso E, Aymerich M, Campo E, Dierssen-Sotos T, Fernandez-Tardon G, Olmedo-Requena R, Gimeno E, Castano-Vinyals G, Aragones N, Kogevinas M, de Sanjose S, Pollan M, Casabonne D (2018) Adherence to the Western, Prudent, and Mediterranean dietary patterns and chronic lymphocytic leukemia in the MCC-Spain study. Haematologica 103(11):1881–1888. https://doi.org/10.3324/haematol.2018.192526
Turati F, Carioli G, Bravi F, Ferraroni M, Serraino D, Montella M, Giacosa A, Toffolutti F, Negri E, Levi F, La Vecchia C (2018) Mediterranean diet and breast cancer risk. Nutrients. https://doi.org/10.3390/nu10030326
Dela Cruz R, Park SY, Shvetsov YB, Boushey CJ, Monroe KR, Le Marchand L, Maskarinec G (2020) Diet quality and breast cancer incidence in the multiethnic cohort. Eur J Clin Nutr. https://doi.org/10.1038/s41430-020-0627-2
Gardeazabal I, Romanos-Nanclares A, Martinez-Gonzalez MA, Castello A, Sanchez-Bayona R, Perez-Gomez B, Razquin C, Aramendia-Beitia JM, Pollan M, Toledo E (2020) Mediterranean dietary pattern is associated with lower incidence of premenopausal breast cancer in the Seguimiento Universidad de Navarra (SUN) Project. Public Health Nutr. https://doi.org/10.1017/s1368980019003835
Lee DH, Fung TT, Tabung FK, Marinac CR, Devore EE, Rosner BA, Ghobrial IM, Colditz GA, Giovannucci EL, Birmann BM (2020) Prediagnosis dietary pattern and survival in patients with multiple myeloma. Int J Cancer. https://doi.org/10.1002/ijc.32928
Boden S, Myte R, Wennberg M, Harlid S, Johansson I, Shivappa N, Hebert JR, Van Guelpen B, Nilsson LM (2019) The inflammatory potential of diet in determining cancer risk; a prospective investigation of two dietary pattern scores. PLoS One 14(4):e0214551. https://doi.org/10.1371/journal.pone.0214551
Bogumil D, Park SY, Le Marchand L, Haiman CA, Wilkens LR, Boushey CJ, Setiawan VW (2019) High-quality diets are associated with reduced risk of hepatocellular carcinoma and chronic liver disease: the multiethnic cohort. Hepatol Commun 3(3):437–447. https://doi.org/10.1002/hep4.1313
Bonaccio M, Di Castelnuovo A, Costanzo S, Gialluisi A, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L (2018) Mediterranean diet and mortality in the elderly: a prospective cohort study and a meta-analysis. Br J Nutr 120(8):841–854. https://doi.org/10.1017/s0007114518002179
Cheng E, Um CY, Prizment A, Lazovich D, Bostick RM (2018) Associations of evolutionary-concordance diet, Mediterranean diet and evolutionary-concordance lifestyle pattern scores with all-cause and cause-specific mortality. Br J Nutr. https://doi.org/10.1017/s0007114518003483
Cheng E, Um CY, Prizment AE, Lazovich D, Bostick RM (2018) Evolutionary-concordance lifestyle and diet and mediterranean diet pattern scores and risk of incident colorectal cancer in iowa women. Cancer Epidemiol Biomarkers Prev 27(10):1195–1202. https://doi.org/10.1158/1055-9965.Epi-17-1184
Lavalette C, Adjibade M, Srour B, Sellem L, Fiolet T, Hercberg S, Latino-Martel P, Fassier P, Deschasaux M, Kesse-Guyot E, Touvier M (2018) Cancer-specific and general nutritional scores and cancer risk: results from the prospective NutriNet-Sante cohort. Cancer Res 78(15):4427–4435. https://doi.org/10.1158/0008-5472.Can-18-0155
Lee DH, Fung TT, Tabung FK, Colditz GA, Ghobrial IM, Rosner BA, Giovannucci EL, Birmann BM (2019) Dietary pattern and risk of multiple myeloma in two large prospective us cohort studies. JNCI Cancer Spectr 3(2):025. https://doi.org/10.1093/jncics/pkz025
Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, Chong D, VoPham T, Meyerhardt JA, Wen D, Giovannucci EL, Chan AT, Zhang X (2019) Dietary patterns and risk of hepatocellular carcinoma among US men and women. Hepatology 70(2):577–586. https://doi.org/10.1002/hep.30362
Neelakantan N, Koh WP, Yuan JM, van Dam RM (2018) Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among Chinese adults. J Nutr 148(8):1323–1332. https://doi.org/10.1093/jn/nxy094
Petimar J, Park YM, Smith-Warner SA, Fung TT, Sandler DP (2019) Dietary index scores and invasive breast cancer risk among women with a family history of breast cancer. Am J Clin Nutr 109(5):1393–1401. https://doi.org/10.1093/ajcn/nqy392
Petimar J, Smith-Warner SA, Fung TT, Rosner B, Chan AT, Hu FB, Giovannucci EL, Tabung FK (2018) Recommendation-based dietary indexes and risk of colorectal cancer in the Nurses' Health Study and Health Professionals Follow-up Study. Am J Clin Nutr 108(5):1092–1103. https://doi.org/10.1093/ajcn/nqy171
Ratjen I, Schafmayer C, di Giuseppe R, Waniek S, Plachta-Danielzik S, Koch M, Nothlings U, Hampe J, Schlesinger S, Lieb W (2017) Postdiagnostic Mediterranean and healthy nordic dietary patterns are inversely associated with all-cause mortality in long-term colorectal cancer survivors. J Nutr 147(4):636–644. https://doi.org/10.3945/jn.116.244129
Sharma I, Roebothan B, Zhu Y, Woodrow J, Parfrey PS, McLaughlin JR, Wang PP (2018) Hypothesis and data-driven dietary patterns and colorectal cancer survival: findings from Newfoundland and Labrador colorectal Cancer cohort. Nutr J 17(1):55. https://doi.org/10.1186/s12937-018-0362-x
Torres Stone RA, Waring ME, Cutrona SL, Kiefe CI, Allison J, Doubeni CA (2017) The association of dietary quality with colorectal cancer among normal weight, overweight and obese men and women: a prospective longitudinal study in the USA. BMJ Open 7(6):e015619. https://doi.org/10.1136/bmjopen-2016-015619
Warensjo Lemming E, Byberg L, Wolk A, Michaelsson K (2018) A comparison between two healthy diet scores, the modified Mediterranean diet score and the Healthy Nordic Food Index, in relation to all-cause and cause-specific mortality. Br J Nutr 119(7):836–846. https://doi.org/10.1017/s0007114518000387
Solans M, Benavente Y, Saez M, Agudo A, Naudin S, Hosnijeh FS, Noh H, Freisling H, Ferrari P, Besson C, Mahamat-Saleh Y, Boutron-Ruault MC, Kuhn T, Kaaks R, Boeing H, Lasheras C, Rodriguez-Barranco M, Amiano P, Huerta JM, Barricarte A, Schmidt JA, Vineis P, Riboli E, Trichopoulou A, Bamia C, Peppa E, Masala G, Agnoli C, Tumino R, Sacerdote C, Panico S, Skeie G, Weiderpass E, Jerkeman M, Ericson U, Spath F, Nilsson LM, Dahm CC, Overvad K, Bolvig AK, Tjonneland A, de Sanjose S, Buckland G, Vermeulen R, Nieters A, Casabonne D (2019) Adherence to the mediterranean diet and lymphoma risk in the european prospective investigation into cancer and nutrition. Int J Cancer 145(1):122–131. https://doi.org/10.1002/ijc.32091
Karavasiloglou N, Pestoni G, Faeh D, Rohrmann S (2019) Post-diagnostic diet quality and mortality in females with self-reported history of breast or gynecological cancers: results from the third national health and nutrition examination survey (NHANES III). Nutrients. https://doi.org/10.3390/nu11112558
Witlox WJA, van Osch FHM, Brinkman M, Jochems S, Goossens ME, Weiderpass E, White E, van den Brandt PA, Giles GG, Milne RL, Huybrechts I, Adami HO, Bueno-de-Mesquita B, Wesselius A, Zeegers MP (2019) An inverse association between the Mediterranean diet and bladder cancer risk: a pooled analysis of 13 cohort studies. Eur J Nutr. https://doi.org/10.1007/s00394-019-01907-8
Schulpen M, Peeters PH, van den Brandt PA (2019) Mediterranean diet adherence and risk of pancreatic cancer: a pooled analysis of two Dutch cohorts. Int J Cancer 144(7):1550–1560. https://doi.org/10.1002/ijc.31872
Schulpen M, Peeters PH, van den Brandt PA (2019) Mediterranean diet adherence and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Gastric Cancer 22(4):663–674. https://doi.org/10.1007/s10120-019-00927-x
Schulpen M, van den Brandt PA (2018) Adherence to the Mediterranean diet and risk of lung cancer in the Netherlands Cohort Study. Br J Nutr 119(6):674–684. https://doi.org/10.1017/s0007114517003737
Schulpen M, van den Brandt PA (2019) Adherence to the Mediterranean diet and risks of prostate and bladder cancer in the netherlands cohort study. Cancer Epidemiol Biomarkers Prev 28(9):1480–1488. https://doi.org/10.1158/1055-9965.Epi-19-0224
Schulpen M, van den Brandt PA (2019) Mediterranean diet adherence and risk of colorectal cancer: the prospective Netherlands Cohort Study. Eur J Epidemiol. https://doi.org/10.1007/s10654-019-00549-8
Sofi F, Macchi C, Abbate R, Gensini GF, Casini A (2014) Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 17(12):2769–2782. https://doi.org/10.1017/s1368980013003169
Fardet A, Rock E (2014) Toward a new philosophy of preventive nutrition: from a reductionist to a holistic paradigm to improve nutritional recommendations. Adv Nutr 5(4):430–446. https://doi.org/10.3945/an.114.006122
Schwingshackl L, Bogensberger B, Hoffmann G (2018) Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 118(1):74–100.e111. https://doi.org/10.1016/j.jand.2017.08.024
Schwingshackl L, Morze J, Hoffmann G (2019) Mediterranean diet and health status: active ingredients and pharmacological mechanisms. Br J Pharmacol. https://doi.org/10.1111/bph.14778
Yang JJ, Yu D, Xiang YB, Blot W, White E, Robien K, Sinha R, Park Y, Takata Y, Lazovich D, Gao YT, Zhang X, Lan Q, Bueno-de-Mesquita B, Johansson I, Tumino R, Riboli E, Tjonneland A, Skeie G, Quiros JR, Johansson M, Smith-Warner SA, Zheng W, Shu XO (2019) Association of dietary fiber and yogurt consumption with lung cancer risk: a pooled analysis. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.4107
Chen S, Chen Y, Ma S, Zheng R, Zhao P, Zhang L, Liu Y, Yu Q, Deng Q, Zhang K (2016) Dietary fibre intake and risk of breast cancer: a systematic review and meta-analysis of epidemiological studies. Oncotarget 7(49):80980–80989. https://doi.org/10.18632/oncotarget.13140
Zhang Z, Xu G, Ma M, Yang J, Liu X (2013) Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology 145(1):113–120.e113. https://doi.org/10.1053/j.gastro.2013.04.001
Fung KYC, Cosgrove L, Lockett T, Head R, Topping DL (2012) A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr 108(5):820–831. https://doi.org/10.1017/S0007114512001948
Do Prado SBR, Castro-Alves VC, Ferreira GF, Fabi JP (2019) Ingestion of non-digestible carbohydrates from plant-source foods and decreased risk of colorectal cancer: a review on the biological effects and the mechanisms of action. Front Nutr. https://doi.org/10.3389/fnut.2019.00072
Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 134(12 Suppl):3479s–3485s. https://doi.org/10.1093/jn/134.12.3479S
Phan MAT, Paterson J, Bucknall M, Arcot J (2018) Interactions between phytochemicals from fruits and vegetables: effects on bioactivities and bioavailability. Crit Rev Food Sci Nutr 58(8):1310–1329. https://doi.org/10.1080/10408398.2016.1254595
Chen H, Liu RH (2018) Potential mechanisms of action of dietary phytochemicals for cancer prevention by targeting cellular signaling transduction pathways. J Agric Food Chem 66(13):3260–3276. https://doi.org/10.1021/acs.jafc.7b04975
Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T (2018) Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr 108(5):1069–1091. https://doi.org/10.1093/ajcn/nqy097
Zhu Y, Sang S (2017) Phytochemicals in whole grain wheat and their health-promoting effects. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201600852
Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T (2016) Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 353:i2716. https://doi.org/10.1136/bmj.i2716
Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Laure Preterre A, Iqbal K, Bechthold A, De Henauw S, Michels N, Devleesschauwer B, Boeing H, Schlesinger S (2018) Food groups and risk of colorectal cancer. Int J Cancer 142(9):1748–1758. https://doi.org/10.1002/ijc.31198
Xu Y, Yang J, Du L, Li K, Zhou Y (2019) Association of whole grain, refined grain, and cereal consumption with gastric cancer risk: a meta-analysis of observational studies. Food Sci Nutr 7(1):256–265. https://doi.org/10.1002/fsn3.878
Vieira AR, Abar L, Chan DSM, Vingeliene S, Polemiti E, Stevens C, Greenwood D, Norat T (2017) Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol Off J Eur Soc Med Oncol 28(8):1788–1802. https://doi.org/10.1093/annonc/mdx171
Demeyer D, Mertens B, De Smet S, Ulens M (2016) Mechanisms linking colorectal cancer to the consumption of (processed) red meat: a review. Crit Rev Food Sci Nutr 56(16):2747–2766. https://doi.org/10.1080/10408398.2013.873886
Kruger C, Zhou Y (2018) Red meat and colon cancer: a review of mechanistic evidence for heme in the context of risk assessment methodology. Food Chem Toxicol 118:131–153. https://doi.org/10.1016/j.fct.2018.04.048
Jeyakumar A, Dissabandara L, Gopalan V (2017) A critical overview on the biological and molecular features of red and processed meat in colorectal carcinogenesis. J Gastroenterol 52(4):407–418. https://doi.org/10.1007/s00535-016-1294-x
Song P, Wu L, Guan W (2015) Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients 7(12):9872–9895. https://doi.org/10.3390/nu7125505
Chiavarini M, Bertarelli G, Minelli L, Fabiani R (2017) Dietary intake of meat cooking-related mutagens (HCAs) and risk of colorectal adenoma and cancer: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu9050514
Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS (2018) Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr 58(9):1428–1447. https://doi.org/10.1080/10408398.2016.1263597
Xu W, Fan H, Han Z, Liu Y, Wang Y, Ge Z (2019) Wine consumption and colorectal cancer risk: a meta-analysis of observational studies. Eur J Cancer Prev 28(3):151–158. https://doi.org/10.1097/cej.0000000000000444
Chen JY, Zhu HC, Guo Q, Shu Z, Bao XH, Sun F, Qin Q, Yang X, Zhang C, Cheng HY, Sun XC (2016) Dose-dependent associations between wine drinking and breast cancer risk—meta-analysis findings. Asian Pac J Cancer Prev 17(3):1221–1233. https://doi.org/10.7314/apjcp.2016.17.3.1221
Wang PL, Xiao FT, Gong BC, Liu FN (2017) Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget 8(58):99013–99023. https://doi.org/10.18632/oncotarget.20918
Martínez-González MÁ, Hershey MS, Zazpe I, Trichopoulou A (2017) Transferability of the mediterranean diet to non-mediterranean countries: what is and what is not the mediterranean diet. Nutrients 9(11):1226. https://doi.org/10.3390/nu9111226
Hatzis CM, Papandreou C, Patelarou E, Vardavas CI, Kimioni E, Sifaki-Pistolla D, Vergetaki A, Kafatos AG (2013) A 50-year follow-up of the seven countries study: prevalence of cardiovascular risk factors, food and nutrient intakes among cretans. Hormones 12(3):379–385. https://doi.org/10.1007/bf03401303
Cooper AJ, Forouhi NG, Ye Z, Buijsse B, Arriola L, Balkau B, Barricarte A, Beulens JW, Boeing H, Buchner FL, Dahm CC, de Lauzon-Guillain B, Fagherazzi G, Franks PW, Gonzalez C, Grioni S, Kaaks R, Key TJ, Masala G, Navarro C, Nilsson P, Overvad K, Panico S, Ramon Quiros J, Rolandsson O, Roswall N, Sacerdote C, Sanchez MJ, Slimani N, Sluijs I, Spijkerman AM, Teucher B, Tjonneland A, Tumino R, Sharp SJ, Langenberg C, Feskens EJ, Riboli E, Wareham NJ (2012) Fruit and vegetable intake and type 2 diabetes: EPIC-interact prospective study and meta-analysis. Eur J Clin Nutr 66(10):1082–1092. https://doi.org/10.1038/ejcn.2012.85
Funding
The research work of Ms Anna Danielewicz and Prof. Katarzyna Przybylowicz is financially supported by the Minister of Science and Higher Education in the range of the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Author information
Authors and Affiliations
Contributions
JM, LS and GH collected the data. JM, and LS carried out data analysis. JM and LS wrote the first draft with contributions from AD, KP, HZ and GH. All authors reviewed and commented on subsequent drafts of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morze, J., Danielewicz, A., Przybyłowicz, K. et al. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur J Nutr 60, 1561–1586 (2021). https://doi.org/10.1007/s00394-020-02346-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02346-6