Abstract
Purpose
Interest in ketogenic diets (KDs) as complementary nutritional treatments for cancer patients is rising, although some skepticism about their safety exists. We, therefore, studied the effects of KDs on quality of life and blood parameters in rectal cancer patients undergoing radio-chemotherapy.
Methods
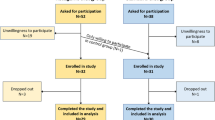
EORTC-QLQ30 questionnaire scores and different metabolic and hormonal blood parameters were obtained prior to, in the middle of and at the end of radiotherapy within the KETOCOMP study (ClinicalTrials.gov Identifier: NCT02516501). A total of 18 patients consuming a KD were compared to 23 patients consuming their standard diet (SD). Baseline-end differences were measured using Wilcoxon tests, and repeated measures analysis was performed using linear mixed effects models.
Results
Eighty-nine percent of patients on the KD reported subjectively feeling good or very good, but roughly half of them rated the daily routine implementation as difficult. Only the SD group experienced significant declines in physical and role functioning, while the KD group improved in role (p = 0.045), emotional (p = 0.018) and social functioning (p = 0.009).Urinary frequency, buttock pain and fatigue significantly increased in the SD group, but to a much lesser extent in the KD group. Several biomarkers of metabolic health (gamma-glutamyl-transpeptidase, triglyceride-glucose index, HDL cholesterol/triglyceride ratio, and free T3) improved in the KD, but not the SD group.
Conclusions
Despite being perceived as difficult to implement by ≈50% of patients, KDs are feasible as complementary therapies alongside radio-chemotherapy and associated with subjective well-being. The hypothesis that they exert beneficial effects on quality of life and metabolic health in rectal cancer patients is supported by our data.
Trial registration
ClinicalTrials.gov identifier NCT02516501, registered Aug 6th 2015.




Similar content being viewed by others
Data availability statement
All data used in this analysis are available from the corresponding author upon reasonable request.
Notes
This threshold was chosen based on the conversion between p values and minimum Bayes factors [52]. Bayes factors (or likelihood ratios in case of simple hypotheses) measure the strength of evidence between two competing hypotheses [70]. In exploratory analyses, a p value of 0.01 corresponds to a minimum Bayes factor of 1/6.5, providing moderate to strong evidence against the null hypothesis [52].
according to our definition of significance (p < 0.01).
References
Miller VJ, Villamena FA, Volek JS (2018) Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab 2018:5157645
Allott EH, Macias E, Sanders S et al (2017) Impact of carbohydrate restriction in the context of obesity on prostate tumor growth in the Hi-Myc transgenic mouse model. Prostate Cancer Prostatic Dis 20:165–171. https://doi.org/10.1038/pcan.2016.73
Hao G-W, Chen Y-S, He D-M et al (2015) Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev 16:2061–2068
Nakamura K, Tonouchi H, Sasayama A, Ashida K (2018) A ketogenic formula prevents tumor progression and cancer cachexia by attenuating systemic inflammation in colon 26 tumor-bearing mice. Nutrients 10:206. https://doi.org/10.3390/nu10020206
Stafford P, Abdelwahab MG, Kim DY et al (2010) The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond) 7:74
Tisdale MJ, Brennan RA, Fearon KC (1987) Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer 56:39–43. https://doi.org/10.1038/bjc.1987.149
Abdelwahab MG, Fenton KE, Preul MC et al (2012) The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE 7:e36197. https://doi.org/10.1371/journal.pone.0036197
Allen BG, Bhatia SK, Buatti JM et al (2013) Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res 19:3905–3913. https://doi.org/10.1158/1078-0432.CCR-12-0287
Aminzadeh-Gohari S, Feichtinger RG, Vidali S, et al (2017) A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget 8:64728–64744. https://doi.org/10.18632/oncotarget.20041
Hopkins BD, Pauli C, Du X et al (2018) Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560:499–503. https://doi.org/10.1038/s41586-018-0343-4
Maeyama M, Tanaka K, Nishihara M et al (2021) Metabolic changes and anti-tumor effects of a ketogenic diet combined with anti-angiogenic therapy in a glioblastoma mouse model. Sci Rep 11:79. https://doi.org/10.1038/s41598-020-79465-x
Ferrere G, Tidjani Alou M, Liu P et al (2021) Ketogenic diet and ketone bodies enhance the anticancer effects of PD1 blockade. JCI Insight 6:e145207. https://doi.org/10.1172/jci.insight.145207
Schmidt M, Pfetzer N, Schwab M et al (2011) Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond) 8:54
Fine EJ, Segal-isaacson CJ, Feinman RD et al (2012) Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition 28:1028–1035. https://doi.org/10.1016/j.nut.2012.05.001
Rieger J, Bähr O, Maurer GD et al (2014) ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol 44:1843–1852. https://doi.org/10.3892/ijo.2014.2382
Voss M, von Mettenheim N, Harter P et al (2020) ERGO2: a prospective randomized trial of calorie restricted ketogenic diet and fasting in addition to re-irradiation for malignant glioma. Int J Radiat Oncol Biol Phys 108:987–995. https://doi.org/10.1016/j.ijrobp.2020.06.021
Tan-Shalaby JL, Carrick J, Edinger K et al (2016) Modified Atkins diet in advanced malignancies—final results of a safety and feasibility trial within the veterans affairs Pittsburgh healthcare system. Nutr Metab (Lond) 13:52. https://doi.org/10.1186/s12986-016-0113-y
Cohen CW, Fontaine KR, Arend RC et al (2018) A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer. J Nutr 148:1253–1260. https://doi.org/10.1093/jn/nxy119
Khodabakhshi A, Akbari ME, Mirzaei HR et al (2019) Feasibility, safety, and beneficial effects of MCT-based ketogenic diet for breast cancer treatment: a randomized controlled trial study. Nutr Cancer 72:627–634. https://doi.org/10.1080/01635581.2019.1650942
Hagihara K, Kajimoto K, Osaga S et al (2020) Promising effect of a new ketogenic diet regimen in patients with advanced cancer. Nutrients 12:1473. https://doi.org/10.3390/nu12051473
Hyde PN, Lustberg MB, Miller VJ et al (2017) Pleiotropic effects of nutritional ketosis: conceptual framework for keto-adaptation as a breast cancer therapy. Cancer Treat Res Commun 12:32–39. https://doi.org/10.1016/j.ctarc.2017.06.001
Lussier DM, Woolf EC, Johnson JL et al (2016) Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 16:10
Bandera-Merchan B, Boughanem H, Crujeiras AB et al (2020) Ketotherapy as an epigenetic modifier in cancer. Rev Endocr Metab Disord 21:509–519. https://doi.org/10.1007/s11154-020-09567-4
Klement RJ (2019) The influence of ketogenic therapy on the 5 R’s of radiobiology. Int J Radiat Biol 95:394–407. https://doi.org/10.1080/09553002.2017.1380330
Klement RJ, Pazienza V (2019) Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina (Kaunas) 55:E84. https://doi.org/10.3390/medicina5504008
Seyfried TN, Flores RE, Poff AM, D’Agostino DP (2014) Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 35:515–527. https://doi.org/10.1093/carcin/bgt480
Klement RJ, Fink MK (2016) Dietary and pharmacological modification of the insulin/IGF-1 system: exploiting the full repertoire against cancer. Oncogenesis 5:e193. https://doi.org/10.1038/oncsis.2016.2
Klement RJ, Brehm N, Sweeney RA (2020) Ketogenic diets in medical oncology: a systematic review with focus on clinical outcomes. Med Oncol 37:14. https://doi.org/10.1007/s12032-020-1337-2
Fearon KC, Borland W, Preston T et al (1988) Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr 47:42–48
Ok JH, Lee H, Chung H-Y et al (2018) The potential use of a ketogenic diet in pancreatobiliary cancer patients after pancreatectomy. Anticancer Res 38:6519–6527. https://doi.org/10.21873/anticanres.13017
Kämmerer U, Klement RJ, Joos FT et al (2021) Low carb and ketogenic diets increase quality of life, physical performance, body composition, and metabolic health of women with breast cancer. Nutrients 13:1029. https://doi.org/10.3390/nu13031029
Burger K (2017) Ärzte warnen Krebspatienten vor Keto-Diäten. In: Süddeutsche Zeitung. https://www.sueddeutsche.de/gesundheit/medizin-aerzte-warnen-krebspatienten-vor-keto-diaeten-1.3687280
Klassen PN, Goldenberg BA, Lambert P et al (2020) Ketogenic and low-sugar diets for patients with cancer: perceptions and practices of medical oncologists in Canada. Support Care Cancer 28:5243–5249
Klement RJ, Feinman RD, Gross EC et al (2017) Need for new review of article on ketogenic dietary regimes for cancer patients. Med Oncol 34:108. https://doi.org/10.1007/s12032-017-0968-4
Klement RJ, Sweeney RA, Gross EC, Champ CE (2019) Problems associated with a highly artificial ketogenic diet: Letter to the Editor Re: van der Louw EJTM, Olieman JF, van den Bemt PMLA, et al. Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: a feasibility and safety. Ther Adv Med Oncol 11:1758835919879268. https://doi.org/10.1177/1758835919879268
Klement RJ (2019) The emerging role of ketogenic diets in cancer treatment. Curr Opin Clin Nutr Metab Care 22:129–134. https://doi.org/10.1097/MCO.0000000000000540
Klement RJ, Schäfer G, Sweeney RA (2020) A ketogenic diet exerts beneficial effects on body composition of cancer patients during radiotherapy: An interim analysis of the KETOCOMP study. J Tradit Complement Med 10:180–187. https://doi.org/10.1016/j.jtcme.2019.03.007
Klement RJ, Champ CE, Kämmerer U et al (2020) Impact of a ketogenic diet intervention during radiotherapy on body composition: III—final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res 22:94. https://doi.org/10.1186/s13058-020-01331-5
Klement RJ, Weigel MM, Sweeney RA (2021) A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr. https://doi.org/10.1016/j.clnu.2021.01.023
Klement RJ, Sweeney RA (2016) Impact of a ketogenic diet intervention during radiotherapy on body composition: II. Protocol of a randomised phase I study (KETOCOMP). Clin Nutr ESPEN 12:e1–e6. https://doi.org/10.1016/j.clnesp.2015.11.001
Jenkins DG, Quintana-Ascencio PF (2020) A solution to minimum sample size for regressions. PLoS ONE 15:e0229345. https://doi.org/10.1371/journal.pone.0229345
Klement RJ, Schäfer G, Sweeney RA (2019) A fatal case of Fournier’s gangrene during neoadjuvant radiotherapy for rectal cancer. Strahlenther Onkol 195:441–446. https://doi.org/10.1007/s00066-018-1401-4
Perini TA, de Oliveira GL, dos Santos OJ, Palha de Oliveira F (2005) Technical error of measurement in anthropometry. Rev Bras Med do Esporte 11:86–90. https://doi.org/10.1590/s1517-86922005000100009
Bosy-Westphal A, Schautz B, Later W et al (2013) What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur J Clin Nutr 67:S14–S21
Bundesärztekammer (2019) Neufassung der Richtlinie der Bundesärztekammer zur Qualitätssicherung Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen. Dtsch Arztebl 116:A–2422. https://doi.org/10.3238/arztebl.2019.rili
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F (2008) The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6:299–304. https://doi.org/10.1089/met.2008.0034
Alizargar J, Hsieh N-C, Wu S-FV (2020) Is the use of triglyceride-glucose (TyG) index to recognize glucose disorders really practical? Eur J Pediatr 179:1169. https://doi.org/10.1007/s00431-020-03642-3
Kämmerer U, Schlatterer C, Knoll G (2012) Krebszellen lieben Zucker—Patienten brauchen Fett, 1st edn. Systemed
Zeevi D, Korem T, Zmora N et al (2015) Personalized nutrition by prediction of glycemic responses. Cell 163:1079–1095. https://doi.org/10.1016/j.cell.2015.11.001
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European organization for research and treatment of cancer QLC-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Fayers P, Aaronson N, Bjordal K et al (2001) The EORTC QOL-C30 scoring manual (3rd Edition). European Organisation for Research and Treatment of Cancer, Brussels
Held L, Ott M (2018) On p -values and Bayes factors. Annu Rev Stat Its Appl 5:393–419
Giesinger JM, Kieffer JM, Fayers PM et al (2016) Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79–88. https://doi.org/10.1016/j.jclinepi.2015.08.007
Stafstrom CE, Rho JM (2012) The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol 3:59. https://doi.org/10.3389/fphar.2012.00059
Klement RJ, Koebrunner PS, Meyer D et al (2021) Impact of a ketogenic diet intervention during radiotherapy on body composition: IV. Final results of the KETOCOMP study for rectal cancer patients. Clin Nutr. https://doi.org/10.1016/j.clnu.2021.05.015
Masino SA, Ruskin DN (2013) Ketogenic diets and pain. J Child Neurol 28:993–1001. https://doi.org/10.1177/0883073813487595
Brenton JN, Banwell B, Bergqvist AGC et al (2019) Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol—Neuroimmunol Neuroinflammation 6:e565. https://doi.org/10.1212/NXI.0000000000000565
Schreck KC, Lwin M, Strowd RE et al (2019) Effect of ketogenic diets on leukocyte counts in patients with epilepsy. Nutr Neurosci 22:522–527. https://doi.org/10.1080/1028415X.2017.1416740
Sürmelıoğlu N, Paydaş S, Karataş Y, Seydaoğlu G (2017) Evaluation of lipid profiles in patients treated with capecitabine. Turkish J Med Sci 47:1206–1209. https://doi.org/10.3906/sag-1607-53
Bar-Sela G, Haim N (2009) Uncontrolled hypertriglyceridemia induced by capecitabine: case report and review of the literature. Cancer Chemother Pharmacol 63:779–782. https://doi.org/10.1007/s00280-008-0799-2
Michie CO, Sakala M, Rivans I et al (2010) The frequency and severity of capecitabine-induced hypertriglyceridaemia in routine clinical practice: a prospective study. Br J Cancer 103:617–621. https://doi.org/10.1038/sj.bjc.6605807
Liu X, He G, Lo K et al (2021) The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med 7:628109. https://doi.org/10.3389/fcvm.2020.628109
Okamura T, Hashimoto Y, Hamaguchi M et al (2020) Triglyceride-glucose index (TyG index) is a predictor of incident colorectal cancer: a population-based longitudinal study. BMC Endocr Disord 20:113. https://doi.org/10.1186/s12902-020-00581-w
Fritz J, Bjørge T, Nagel G et al (2020) The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol 49:193–204. https://doi.org/10.1093/ije/dyz053
Lahm H, Suardet L, Laurent PL et al (1992) Growth regulation and co-stimulation of human colorectal cancer cell lines by insulin-like growth factor I, II and transforming growth factor α. Br J Cancer 65:341–346. https://doi.org/10.1038/bjc.1992.69
Palmqvist R, Hallmans G, Rinaldi S et al (2002) Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut 50:642–646. https://doi.org/10.1136/gut.50.5.642
Murphy N, Carreras-Torres R, Song M et al (2020) Circulating levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 associate with risk of colorectal cancer based on serologic and Mendelian randomization analyses. Gastroenterology 158:1300-1312.e20. https://doi.org/10.1053/j.gastro.2019.12.020
Khodabakhshi A, Akbari ME, Mirzaei HR et al (2020) Effects of ketogenic metabolic therapy on patients with breast cancer: a randomized controlled clinical trial. Clin Nutr 40:751–758. https://doi.org/10.1016/j.clnu.2020.06.028
Koutnik AP, Poff AM, Ward NP et al (2020) Ketone bodies attenuate wasting in models of atrophy. J Cachexia Sarcopenia Muscle 11:973–996. https://doi.org/10.1002/jcsm.12554
Bandyopadhyay PS, Brittan G Jr, Taper ML (2016) Belief, evidence, and uncertainty: problems of epistemic inference, 1st edn. Springer International Publishing, Basel
Acknowledgements
We thank Petra Koebrunner, Kelly Krage and Nanina Brehm for helping us to collect the data.
Funding
This study received no specific funding.
Author information
Authors and Affiliations
Contributions
RJK: conceptualization, data curation, formal analysis, supervision, and writing—original draft. DM: resources and writing—review and editing. SK: resources and writing—review and editing. RAS: conceptualization, supervision, resources, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
RJK has received an honorarium from the company vitaflo for giving a talk about the objectives and preliminary results of the KETOCOMP study. RJK and RAS are occasionally on a ketogenic diet. No other potential conflicts of interest associated with this research exist or any of the authors.
Ethical approval
This study has been approved by the ethics committee of the Bavarian Medical Association (Landesärztekammer Bayern) under reference number 15025 and registered on 6 Aug 2015 under ClinicalTrials.gov identifier NCT02516501 (URL: https://clinicaltrials.gov/ct2/show/NCT02516501).
Consent to participate
All study subjects gave their written informed consent to participate in the study with the option to withdraw at any time.
Consent for publication
All study subjects gave their written informed consent that their data could be used for publications in anonymized form.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klement, R.J., Meyer, D., Kanzler, S. et al. Ketogenic diets consumed during radio-chemotherapy have beneficial effects on quality of life and metabolic health in patients with rectal cancer. Eur J Nutr 61, 69–84 (2022). https://doi.org/10.1007/s00394-021-02615-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02615-y