Abstract
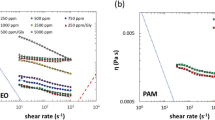
We report a stress-controlled microfluidic shear viscometer with flow visualization aided by smartphone technology. The method involves driving the fluid into a microchannel at constant pressure and using the smartphone camera to track the fluid front in a glass capillary attached to the microchannel. We find that videos of interface propagation from the smartphone are of sufficient resolution that accurate pressure drop-flow rate relations can be determined to quantify the viscosity curves for complex fluids. We demonstrate that this simple ‘iCapillary’ device measures the shear viscosity of Newtonian and polymeric fluids over a broad range of shear rates (10–10,000 s−1) that is in quantitative agreement with rotational rheometry. We further show that the simplicity of the iCapillary device allows for parallel analysis of viscosity of several samples. We performed multiplexed measurements of concentration dependence of high shear rate viscosity of globular protein solutions, and the results are in good agreement with models of suspension rheology as well as prior experimental data. Our approach is unique, since no on-chip sensing element is required other than the smartphone camera. This sensor-less approach offers the potential to create inexpensive and disposable devices for point-of-care rheology of complex fluids and biological samples.








Similar content being viewed by others
References
Allmendinger A, Fischer S, Huwyler B, Mahler HC, Schwarb E, Zarraga IE, Mueller R (2014) Rheological characterization and injection forces of concentrated protein formulations: an alternative predictive model for non-Newtonian solutions. Eur J Pharm Biopharm 87(2):318–328
Bair S, Jarzynski J, Winer WO (2001) The temperature, pressure and time dependence of lubricant viscosity. Tribol Int 34(7):461–468
Batchelor GK, Green JT (1972) The hydrodynamic interaction of two small freely-moving spheres in a linear flow field. J Fluid Mech 56(2):375–400
Brownsey GJ, Noel TR, Parker R, Ring SG (2003) The glass transition behavior of the globular protein bovine serum albumin. Biophys J 85(6):3943–3950
Bruss H (2008) Theoretical microfluidics. Oxford University Press, Oxford
Buchmann, S. (2001). Main cosmetic vehicles. Handbook of Cosmetic Science and Technology. A. O. Barel, M. Paye and H. I. Maibach. New York, Marcel Dekker, Inc.: 145–171.
Burckbuchler V, Mekhloufi G, Giteau AP, Grossiord JL, Huille S, Agnely F (2010) Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur J Pharm Biopharm 76(3):351–356
Cartas-Ayala M, Karnik R (2013) Time limitations and geometrical parameters in the design of microfluidic comparators. Microfluid Nanofluid 17(2):359–373
de Carvalho MJS, Seidl PR, Belchior CRP, Sodre JR (2010) Lubricant viscosity and viscosity improver additive effects on diesel fuel economy. Tribol Int 43(12):2298–2302
Dörr A, Sadiki A, Mehdizadeh A (2013) A discrete model for the apparent viscosity of polydisperse suspensions including maximum packing fraction. Journal of Rheology (1978-present) 57(3):743–765
Finlayson-Pitts BJ, Wingen LM, Sumner AL, Syomin D, Ramazan KA (2003) The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: an integrated mechanism. Phys Chem Chem Phys 5(2):223–242
Galambos P, Forster F (1998) An Optical Microfluidic Viscosmeter. ASME Int. Mech.Eng.Cong.&Exp 66:187–191
Galindo-Rosales FJ, Alves MA, Oliveira MSN (2013) Microdevices for extensional rheometry of low viscosity elastic liquids: a review. Microfluid Nanofluid 14:1–19
Genovese DB, Lozano JE, Rao MA (2007) The rheology of colloidal and Noncolloidal food dispersions. J Food Sci 72(2):R11–R20
Gervais T, El-Ali J, Gunther A, Jensen KF (2006) Flow-induced deformation of shallow microfluidic channels. Lab Chip 6(4):500–507
Goswami S, Wang W, Arakawa T, Ohtake S (2013) Developments and challenges for mAb-based therapeutics. Antibodies 2:452–500
Guillot P, Colin A (2014) Determination of the flow curve of complex fluids using the Rabinowitsch- Mooney equation in sensorless microrheometer. Microfluid. Nanofluid. 17:605–611
Guillot P, Pascal P, Salmo JB, Mathieu B, Colin A (2006) Viscosimeter on a microfluidic chip. Langmuir 22:6438–6445
Han Z, Tang X, Zheng B (2007) A PDMS viscometer for microliter Newtonian fluid. J Micromech Microeng 17(9):1828–1834
Hudson SD, Sarangapani P, Pathak JA, Migler KB (2015) A microliter capillary rheometer for characterization of protein solutions. J Pharm Sci 104(2):678–685
Hurth C, Klein K, van Nimwegen L, Korn R, Vijayaraghavan K, Zenhausern F (2011) Clinical diagnostic of pleural effusions using a high-speed viscosity measurement method. J Appl Phys 110(3)
Ikeda S, Nishinari K (2000) Intermolecular forces in bovine serum albumin solutions exhibiting solid-like mechanical behaviors. Biomacromolecules 1(4):757–763
Kontopoulou M (2011) Applied polymer rheology:polymeric fluids with industrial applications. Wiley, New York
Krieger IM, Dougherty TJ (1959) A mechanism for non-Newtonian flow in suspensions of rigid spheres. Transactions of The Society of Rheology (1957–1977) 3(1):137–152
Lee J, Tripathi A (2005) Intrinsic viscosity of polymers and biopolymers measured by microchip. Anal Chem 77(22):7137–7147
Livak-Dahl E, Lee J, Burns MA (2013) Nanoliter droplet viscometer with additive free operation. Lab Chip 13(2):297–301
Macosko CW (1994) Rheology: principles, measurements and applications. Wiley-VCH, New York
Mewis J, Wagner NJ (2012) Colloidal suspension rheology. Cambridge University Press, Cambridge
Mueller S, Llewellin E, Mader H (2009) The rheology of suspensions of solid particles. Proceedings of the Royal Society of. London A, Mathematical, Physical and Engineering Sciences, The Royal Society
Pan, L. and P. E. Arratia (2012). A high-shear, low Reynolds number microfluidic rheometer. Microfluidics and Nanofluidics(1613–4982).
Peters T (1996) All about albumin: biochemistry, genetics, and medical applications. Academic Press, San Diego
Pipe CJ, Majmudar TS, McKinley GH (2008) High shear rate viscometry. Rheol Acta 47:621–642
Purwar S, Lim JK, Mauger JW, Howard SA (1988) Measuring viscosity of pharmaceutical and cosmetic semisolids using normal stress. J Soc Cosmet Chem 39(4):241–258
Rao MA (2007) Rheology of fluid and semisolid foods: principles and applications. Springer, New York
Santoyo E, Santoyo-Gutierrez S, Garcia A, Espinosa G, Moya SL (2001) Rheological property measurement of drilling fluids used in geothermal wells. Appl Therm Eng 21:283–302
Sarangapani PS, Hudson SD, Jones RL, Douglas JF, Pathak JA (2015) Critical examination of the colloidal particle model of globular proteins. Biophys J 108(3):724–737
Sharma V, Jaishankar A, Wang YC, McKinley GH (2011) Rheology of globular proteins: apparent yield stress, high shear rate viscosity and interfacial viscoelasticity of bovine serum albumin solutions. Soft Matter 7(11):5150–5160
Shire SJ, Shahrokh Z, Liu J (2004) Challenges in the development of high protein concentration formulations. J Pharm Sci 93(6):1390–1402
Solomon D, Vanapalli S (2014) Multiplexed microfluidic viscometer for high-throughput complex fluid rheology. Microfluid Nanofluid 16(4):677–690
Srivastava N, Davenport RD, Burns MA (2005) Nanoliter viscometer for analyzing blood plasma and other liquid samples. Anal Chem 77:383–392
Tabbernor, A. (1988). Rheology of printing inks. The Printing Ink Manual. R. H. Leach, C. Armstrong, J. F. Brown et al. US, Springer: 666–698.
Vanapalli SA, Islam MT, Solomon MJ (2005) Scission-induced bounds on maximum polymer drag reduction in turbulent flow. Physics of Fluids, 17(9):095108
Vanapalli SA, van den Ende D, Duits MHG, Mugele F (2007) Scaling of interface displacement in a microfluidic comparator. Appl Phys Lett 90:114109
Vargaftik NB, Volkov BN, Voljak LD (1983) International tables of the surface-tension of water. J Phys Chem Ref Data 12(3):817–820
Xia YN, Whitesides GM (1998) Soft lithography. Angewandte Chemie-International Edition 37(5):551–575
Yu GR, Zhao DC, Wen L, Yang SD, Chen XC (2012) Viscosity of ionic liquids: database, observation, and quantitative structure-property relationship analysis. AICHE J 58(9):2885–2899
Acknowledgments
We thank William S. Wang and Biddut Bhattacharjee for useful discussions and Prof. Rajesh Khare for access to the rheometer. We acknowledge the donors of the American Chemical Society–Petroleum Research Fund (Grant No. 50521-DNI9) for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This technology has been licensed to Neofluidics LLC. Deepak Solomon is an equity holder and employee of Neofluidics LLC. Deepak Solomon and Siva Vanapalli qualify to receive royalty distributions from patents assigned to Texas Tech University and licensed for commercial development to Neofluidics LLC.
Appendix
Appendix
Wall shear stress relation for the microchannel in the iCapillary device
To calculate the viscosity for a Newtonian fluid, we use a resistive network approach similar to electrical circuits, where the relation between pressure drop and flow rate is specified using the analogy of Ohm’s law. A schematic representation of the iCapillary device is shown in Fig. 1, which also depicts the pressures at different sections of the device. The pressure drop of a fluid flowing through a conduit can be determined from the product of the hydrodynamic resistance of the fluid (R) and the volumetric flow rate (Q). The hydrodynamic resistance is a function of fluid viscosity and conduit dimensions.
For the microchannel, the pressure drop and flow rate relation is given by
where P c , P 1 and R c are the inlet pressure, outlet pressure and hydraulic resistance of the microchannel respectively.
Likewise for the glass capillary, the pressure drop and flow rate relation is given by
where P int , P h1 and R g are the internal pressure, hydrostatic head and the hydraulic resistance of the glass capillary respectively.
Finally, the Laplace pressure jump (P L ) across the air-water interface is given by
where P atm is the atmospheric pressure, taken as zero.
Since R g < < R c , we obtain the pressure drop across the microchannel (ΔP) as
Taking a control volume inside the microchannel and balancing the forces due to pressure and wall shear stress gives
In Eq. (6), L ch , w and h are the length, width and height of the microchannel respectively. Using Eq. (5) in Eq. (6), we thus obtain the wall shear stress experienced by the microchannel in the iCapillary device as
Rights and permissions
About this article
Cite this article
Solomon, D.E., Abdel-Raziq, A. & Vanapalli, S.A. A stress-controlled microfluidic shear viscometer based on smartphone imaging. Rheol Acta 55, 727–738 (2016). https://doi.org/10.1007/s00397-016-0940-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-016-0940-9