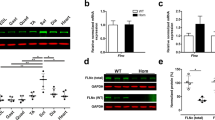
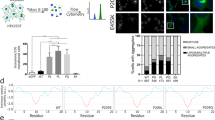
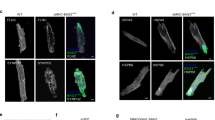
Abstract
Mutations in the co-chaperone Bcl2-associated athanogene 3 (BAG3) can cause myofibrillar myopathy (MFM), a childhood-onset progressive muscle disease, characterized by the formation of protein aggregates and myofibrillar disintegration. In contrast to other MFM-causing proteins, BAG3 has no direct structural role, but regulates autophagy and the degradation of misfolded proteins. To investigate the mechanism of disease in BAG3-related MFM, we expressed wild-type BAG3 or the dominant MFM-causing BAG3 (BAG3P209L) in zebrafish. Expression of the mutant protein results in the formation of aggregates that contain wild-type BAG3. Through the stimulation and inhibition of autophagy, we tested the prevailing hypothesis that impaired autophagic function is responsible for the formation of protein aggregates. Contrary to the existing theory, our studies reveal that inhibition of autophagy is not sufficient to induce protein aggregation. Expression of the mutant protein, however, did not induce myofibrillar disintegration and we therefore examined the effect of knocking down Bag3 function. Loss of Bag3 resulted in myofibrillar disintegration, but not in the formation of protein aggregates. Remarkably, BAG3P209L is able to rescue the myofibrillar disintegration phenotype, further demonstrating that its function is not impaired. Together, our knockdown and overexpression experiments identify a mechanism whereby BAG3P209L aggregates form, gradually reducing the pool of available BAG3, which eventually results in BAG3 insufficiency and myofibrillar disintegration. This mechanism is consistent with the childhood onset and progressive nature of MFM and suggests that reducing aggregation through enhanced degradation or inhibition of nucleation would be an effective therapy for this disease.








Similar content being viewed by others
References
Arimura T, Ishikawa T, Nunoda S et al (2011) Dilated cardiomyopathy-associated BAG3 mutations impair Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum Mutat 32:1481–1491. doi:10.1002/humu.21603
Arndt V, Dick N, Tawo R et al (2010) Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 20:143–148. doi:10.1016/j.cub.2009.11.022
Berghmans S, Butler P, Goldsmith P et al (2008) Zebrafish based assays for the assessment of cardiac, visual and gut function––potential safety screens for early drug discovery. J Pharmacol Toxicol Methods 58:59–68. doi:10.1016/j.vascn.2008.05.130
Boglev Y, Badrock AP, Trotter AJ et al (2013) Autophagy induction is a Tor- and Tp53-independent cell survival response in a zebrafish model of disrupted ribosome biogenesis. PLoS Genet 9:e1003279. doi:10.1371/journal.pgen.1003279
Bross P, Jespersen C, Jensen TG et al (1995) Effects of two mutations detected in medium chain acyl-CoA dehydrogenase (MCAD)-deficient patients on folding, oligomer assembly, and stability of MCAD enzyme. J Biol Chem 270:10284–10290
Claeys KG, Ven PFM, Behin A et al (2009) Differential involvement of sarcomeric proteins in myofibrillar myopathies: a morphological and immunohistochemical study. Acta Neuropathol 117:293–307. doi:10.1007/s00401-008-0479-7
Clemen CS, Herrmann H, Strelkov SV, Schröder R (2013) Desminopathies: pathology and mechanisms. Acta Neuropathol 125:47–75. doi:10.1007/s00401-012-1057-6
Feldman AM, Begay RL, Knezevic T et al (2014) Decreased levels of BAG3 in a family with a rare variant and in idiopathic dilated cardiomyopathy. J Cell Physiol 229:1697–1702. doi:10.1002/jcp.24615
Franaszczyk M, Bilinska ZT, Sobieszcza Ska-Ma Ek MG et al (2014) The BAG3 gene variants in Polish patients with dilated cardiomyopathy: four novel mutations and a genotype-phenotype correlation. J Transl Med 12:192. doi:10.1186/1479-5876-12-192
Fuchs M, Poirier DJ, Seguin SJ et al (2010) Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J 425:245–255. doi:10.1042/BJ20090907
Fürst DO, Goldfarb LG, Kley RA et al (2013) Filamin C-related myopathies: pathology and mechanisms. Acta Neuropathol 125:33–46. doi:10.1007/s00401-012-1054-9
Gámez A, Pérez B, Ugarte M, Desviat LR (2000) Expression analysis of phenylketonuria mutations. Effect on folding and stability of the phenylalanine hydroxylase protein. J Biol Chem 275:29737–29742. doi:10.1074/jbc.M003231200
Goldfarb LG, Park KY, Cervenáková L et al (1998) Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat Genet 19:402–403. doi:10.1038/1300
Higashijima S, Okamoto H, Ueno N et al (1997) High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol 192:289–299
Hishiya A, Salman MN, Carra S et al (2011) BAG3 directly interacts with mutated alphaB-crystallin to suppress its aggregation and toxicity. PLoS One 6:e16828. doi:10.1371/journal.pone.0016828
Homma S, Iwasaki M, Shelton GD et al (2006) BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol 169:761–773. doi:10.2353/ajpath.2006.060250
Hunt L, Atherton J, McGaughran J (2014) Dilated cardiomyopathy––three brothers and a BAG3 mutation. Heart Lung Circ 23(Suppl 2):e11. doi:10.1016/j.hlc.2014.07.029
Kimmel CB, Ballard WW, Kimmel SR et al (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. doi:10.1002/aja.1002030302
Kley RA, Serdaroglu-Oflazer P, Leber Y et al (2012) Pathophysiology of protein aggregation and extended phenotyping in filaminopathy. Brain 135:2642–2660. doi:10.1093/brain/aws200
Kwan KM, Fujimoto E, Grabher C et al (2007) The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236:3088–3099. doi:10.1002/dvdy.21343
Lee H, Cherk S, Chan S et al (2012) BAG3-related myofibrillar myopathy in a Chinese family. Clin Genet 81:394–398. doi:10.1111/j.1399-0004.2011.01659.x
Lee JH, Takahashi T, Yasuhara N et al (1999) Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene 18:6183–6190. doi:10.1038/sj.onc.1203043
Lünemann JD, Schmidt J, Schmid D et al (2007) Beta-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann Neurol 61:476–483. doi:10.1002/ana.21115
Miles LB, Verkade H (2014) TA-cloning vectors for rapid and cheap cloning of zebrafish transgenesis constructs. Zebrafish 11:281–282. doi:10.1089/zeb.2013.0954
Norton N, Li D, Rieder MJ et al (2011) Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am J Hum Genet 88:273–282. doi:10.1016/j.ajhg.2011.01.016
Odgerel Z, Sarkozy A, Lee H-S et al (2010) Inheritance patterns and phenotypic features of myofibrillar myopathy associated with a BAG3 mutation. Neuromuscul Disord 20:438–442. doi:10.1016/j.nmd.2010.05.004
Olivé M, Kley RA, Goldfarb LG (2013) Myofibrillar myopathies: new developments. Curr Opin Neurol 26:527–535. doi:10.1097/WCO.0b013e328364d6b1
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969–1978. doi:10.1093/brain/awm318
Pfeffer G, Barresi R, Wilson IJ et al (2013) Titin founder mutation is a common cause of myofibrillar myopathy with early respiratory failure. J Neurol Neurosurg Psychiatr. doi:10.1136/jnnp-2012-304728
Ravikumar B, Vacher C, Berger Z et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595. doi:10.1038/ng1362
Ruparelia AA, Zhao M, Currie PD, Bryson-Richardson RJ (2012) Characterization and investigation of zebrafish models of filamin-related myofibrillar myopathy. Hum Mol Genet. doi:10.1093/hmg/dds231
Ruparelia A, Vaz R, Bryson-Richardson R (2012) Myofibrillar myopathies and the Z-disk associated proteins. In: Cseri J (ed) Skeletal muscle––from myogenesis to clinical relations. InTech, Croatia, pp 317–358
Schessl J, Zou Y, McGrath MJ et al (2008) Proteomic identification of FHL1 as the protein mutated in human reducing body myopathy. J Clin Invest 118:904–912. doi:10.1172/JCI34450
Schröder R, Kunz WS, Rouan F et al (2002) Disorganization of the desmin cytoskeleton and mitochondrial dysfunction in plectin-related epidermolysis bullosa simplex with muscular dystrophy. J Neuropathol Exp Neurol 61:520–530
Selcen D, Engel AG (2004) Mutations in myotilin cause myofibrillar myopathy. Neurology 62:1363–1371
Selcen D, Engel AG (2005) Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann Neurol 57:269–276. doi:10.1002/ana.20376
Selcen D, Engel AG (2011) Myofibrillar myopathies. Handb Clin Neurol 101:143–154. doi:10.1016/B978-0-08-045031-5.00011-6
Selcen D, Muntoni F, Burton BK et al (2009) Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol 65:83–89. doi:10.1002/ana.21553
Takayama S, Xie Z, Reed JC (1999) An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem 274:781–786
Ulbricht A, Arndt V, Höhfeld J (2013) Chaperone-assisted proteostasis is essential for mechanotransduction in mammalian cells. Commun Integr Biol 6:e24925. doi:10.4161/cib.24925
Ulbricht A, Eppler FJ, Tapia VE et al (2013) Cellular Mechanotransduction Relies on Tension-Induced and Chaperone-Assisted Autophagy. Curr Biol. doi:10.1016/j.cub.2013.01.064
Vicart P, Caron A, Guicheney P et al (1998) A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20:92–95. doi:10.1038/1765
Vorgerd M, van der Ven PFM, Bruchertseifer V et al (2005) A mutation in the dimerization domain of filamin c causes a novel type of autosomal dominant myofibrillar myopathy. Am J Hum Genet 77:297–304. doi:10.1086/431959
Wang J, Shaner N, Mittal B et al (2005) Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskelet 61:34–48. doi:10.1002/cm.20063
Williams A, Sarkar S, Cuddon P et al (2008) Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol 4:295–305. doi:10.1038/nchembio.79
Winter L, Wiche G (2013) The many faces of plectin and plectinopathies: pathology and mechanisms. Acta Neuropathol 125:77–93. doi:10.1007/s00401-012-1026-0
Wynn RM, Davie JR, Chuang JL et al (1998) Impaired assembly of E1 decarboxylase of the branched-chain alpha-ketoacid dehydrogenase complex in type IA maple syrup urine disease. J Biol Chem 273:13110–13118
Acknowledgments
The authors wish to thank Dr. Peter van der Ven and Prof. Dieter Fürst for generously providing the full-length wild-type Filamin C construct, and the staff of the Monash FishCore facility for zebrafish care and maintenance. This work was supported by a Monash University Science Faculty Dean’s scholarship to A.A.R and an NHMRC discovery project Grant (#1010110). The contents of this manuscript are solely the responsibility of the authors and do not reflect the views of NHMRC.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruparelia, A.A., Oorschot, V., Vaz, R. et al. Zebrafish models of BAG3 myofibrillar myopathy suggest a toxic gain of function leading to BAG3 insufficiency. Acta Neuropathol 128, 821–833 (2014). https://doi.org/10.1007/s00401-014-1344-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1344-5