Abstract
Most magmatic-hydrothermal Cu deposits are genetically linked to arc magmas. However, most continental or oceanic arc magmas are barren, and hence new methods have to be developed to distinguish between barren and mineralised arc systems. Source composition, melting conditions, the timing of S saturation and an initial chalcophile element-enrichment represent important parameters that control the potential of a subduction setting to host an economically valuable deposit. Brothers volcano in the Kermadec island arc is one of the best-studied examples of arc-related submarine magmatic-hydrothermal activity. This study, for the first time, compares the chemical and mineralogical composition of the Brothers seafloor massive sulphides and the associated dacitic to rhyolitic lavas that host the hydrothermal system. Incompatible trace element ratios, such as La/Sm and Ce/Pb, indicate that the basaltic melts from L’Esperance volcano may represent a parental analogue to the more evolved Brothers lavas. Copper-rich magmatic sulphides (Cu > 2 wt%) identified in fresh volcanic glass and phenocryst phases, such as clinopyroxene, plagioclase and Fe–Ti oxide suggest that the surrounding lavas that host the Brothers hydrothermal system represent a potential Cu source for the sulphide ores at the seafloor. Thermodynamic calculations reveal that the Brothers melts reached volatile saturation during their evolution. Melt inclusion data and the occurrence of sulphides along vesicle margins indicate that an exsolving volatile phase extracted Cu from the silicate melt and probably contributed it to the overlying hydrothermal system. Hence, the formation of the Cu-rich seafloor massive sulphides (up to 35.6 wt%) is probably due to the contribution of Cu from a bimodal source including wall rock leaching and magmatic degassing, in a mineralisation style that is hybrid between Cyprus-type volcanic-hosted massive sulphide and subaerial epithermal–porphyry deposits.
Similar content being viewed by others
Introduction
Magmatic-hydrothermal systems along convergent plate margins host some of the Earth’s most important porphyry Cu–Mo and epithermal Au–Ag–Te deposits (Seedorff et al. 2005; Simmons 2005; Sillitoe 2010). Most of these metals are considered to be derived from fluids of magmatic origin that exsolved from mid- to upper crustal magma chambers (Richards 2011). Recent studies have shown that submarine hydrothermal systems associated with island arc volcanism can be affected by a magmatic volatile component similar to their subaerial counterparts (de Ronde et al. 2005, 2014). Brothers volcano in the Kermadec island arc represents one of the best-studied examples of arc-related submarine magmatic-hydrothermal activity; and fluid and sulphide chemistry suggest a magmatic volatile contribution (de Ronde et al. 2005, 2011; Keith et al. 2016a). In addition to a magmatic volatile signature, acid-sulphate (advanced argillic) alteration, sulphosalts (e.g., enargite, Cu3AsS4) typical for high-sulphidation conditions (Einaudi et al. 2003) and high contents of economically important metals (e.g., Cu and Au) in some submarine arc and back-arc hydrothermal systems imply that they may be comparable to epithermal–porphyry deposits on land, such as the world-class Far Southeast-Lepanto Cu–Au deposits (Philippines; Hedenquist et al. 1998). This includes, for example, the magmatic-hydrothermal systems of Brothers volcano (Kermadec arc; de Ronde et al. 2005; Berkenbosch et al. 2012) and Kolumbo volcano (Hellenic arc; Kilias et al. 2013, 2016), as well as SuSu Knolls in the Manus back-arc basin (Craddock and Bach 2010; Craddock et al. 2010; Yeats et al. 2014; Thal et al. 2016). Hence, arc-hosted hydrothermal systems are characteristically distinct in terms of their chemical and mineralogical composition compared to those along mid-ocean ridges, which were interpreted to be the classic modern analogues of volcanic-hosted massive sulphide (VHMS) deposits mined on land (Hannington et al. 1998, 2011; de Ronde et al. 2014; Keith et al. 2016b).
High bulk ore contents of certain metals and semi-metals, such as Cu (up to 27 wt%), Au (up to 57 ppm), Ag (up to 2010 ppm) and Te (up to 53 ppm) at PACMANUS and SuSu Knolls (Manus Basin; Moss and Scott 2001) have attracted attention for potential future submarine mining operations (Gena 2013). Consequently, it is important to improve our understanding of the ore-forming processes in subduction zone-related magmatic-hydrothermal systems and to identify chemical and mineralogical parameters for exploration and their economic evaluation.
A key process for the formation of a Cu-rich magmatic-hydrothermal ore deposit is the loss of magmatic volatiles prior to the formation of immiscible sulphide liquids. In contrast, early sulphide segregation, i.e., prior to degassing, would extract most chalcophile metals including Cu and Au from the melt leading to a barren arc system (Sun et al. 2004; Jenner et al. 2010; Richards 2011; Park et al. 2015; Fontboté et al. 2017). This idea appears to be supported by previous studies, which suggest that aqueous S- and Cl-bearing magmatic volatiles are the main source and transport medium for Cu in submarine hydrothermal arc systems, such as Brothers volcano (de Ronde et al. 2005; Berkenbosch et al. 2012; Gruen et al. 2014). Therefore, economic mineralisation within an island arc can be controlled by sulphide liquid immiscibility during silicate melt evolution, which is highly sensitive to changes in pressure, temperature, oxygen fugacity (fO2), the degree of fractional crystallisation and the initial Fe and S contents of the melt (Mavrogenes and O’Neill 1999; Jenner et al. 2010; Yang et al. 2014; Keith et al. 2017). Arc magmas characteristically show higher fO2 (up to FMQ + 2) and H2O contents (up to 6–8 wt%) and are enriched in incompatible trace elements and volatiles (e.g., S and Cl) compared to mid-ocean ridge magmas (Wallace 2005; Richards 2011; Kelley and Cottrell 2012; Lee et al. 2012). This discrepancy results in higher S concentrations at sulphide saturation (Fortin et al. 2015), and hence sulphide segregation typically occurs later during magmatic differentiation, perhaps triggered by Fe–Ti oxide fractionation and decreasing fO2 (Jenner et al. 2010; Keith et al. 2017; Patten et al. 2017). However, distinguishing these different processes and parameters is still challenging and often ambiguous (Hedenquist and Lowenstern 1994) and our knowledge about the interaction between the magmatic and hydrothermal system and its control on the composition of associated ore deposits is limited.
This study combines the chemical and mineralogical composition of magmatic and hydrothermal sulphides with silicate glass and melt inclusion data for the first time to constrain the source characteristics and main enrichment processes of Cu in an island arc-hosted magmatic-hydrothermal system.
Geological setting and sample localities
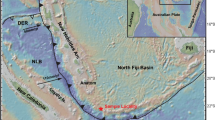
Island arc systems with a submarine expression have a combined length of about 21,700 km (93% in the Pacific Ocean; de Ronde et al. 2014). Brothers volcano is part of the ~ 1200 km long Kermadec island arc, northeast of New Zealand (Fig. 1a), which post-dates the earliest phase of Havre Trough back-arc rifting (Wright et al. 1996, 1998). The elongated volcanic edifice of Brothers is 13 km long, 8 km wide and strikes NW–SE at a water depth of 2200 mbsl (de Ronde et al. 2005). The caldera floor (1850 mbsl) has a basal dimension of 3 to 3.5 km and is surrounded by a 350–450 m high caldera wall (Fig. 1b; Wright et al. 1998). The caldera hosts a resurgent dome rising to a water depth of 1300 mbsl at its summit (Fig. 1b; Wright and Gamble 1999; de Ronde et al. 2001). Hence, at least two stages of magmatic activity can be distinguished at Brothers volcano including (1) an effusive incremental caldera-forming eruption (Wright and Gamble 1999) and (2) the subsequent formation of two younger volcanic cones (Embley et al. 2012). The Brothers volcanic rocks range in composition from dacite to rhyolite (Haase et al. 2006). In addition, one basaltic andesite sample was recovered from Brothers Ridge, a young SW-NE striking mafic dyke beneath the Brothers caldera (Wysoczanski et al. 2012).
a Map of the Kermadec island arc showing the location of Brothers volcano, modified after de Ronde et al. (2011). b Detailed bathymetric map of Brothers volcano with the dredge locations (dashed boxes) of research cruise SO135 (Stoffers et al. 1998) for the samples investigated in this study from the NW caldera and the lower and upper cone complex. Map modified after Baker et al. (2012). LC lower cone, NWC NW caldera, SEC SE caldera, UC upper cone, WC western caldera (figures in colour can be found in the web version of the article)
Several hydrothermal fields were distinguished including hydrothermal venting of evolved seawater derived fluids with limited magmatic contribution at the NW caldera wall and magmatic volatile-dominated systems at the Brothers cone complex (Fig. 1b; de Ronde et al. 2005, 2011; Baker et al. 2012; Caratori Tontini et al. 2012). Hydrodynamic modelling calculations by Gruen et al. (2014) suggest that a 3 × 2 km2 sized magma chamber at ~ 2.5 km depth below the seafloor may represent the heat source for the Brothers magmatic-hydrothermal system. In 1996 the R/V Tangaroa dredged the first massive sulphide-bearing samples suggesting that Brothers volcano hosts a magmatic-hydrothermal system (Wright et al. 1998). This finding lead to the discovery of an active black smoker field at the caldera floor in 1998 during R/V Sonne cruise SO135 (Stoffers et al. 1998). Two types of massive sulphide mineralisation were distinguished including (1) high temperature (> 300 °C) chalcopyrite-dominated Cu–Fe-rich samples with up to 35.6 wt% Cu and (2) low temperature (< 120 °C) Zn–Fe-rich samples with abundant sphalerite reaching Zn concentrations of up to 18.8 wt% in the bulk ore (Wright et al. 1998; de Ronde et al. 2011; Berkenbosch et al. 2012). The samples presented in this study were dredged from Brothers volcano during R/V Sonne cruise SO135 (Table 1; Stoffers et al. 1998) and include volcanic rocks that are representative for the caldera-forming event (n = 19) and the formation of the cone complex (n = 6, Fig. 1b).
Methods
Electron probe microanalysis
In a previous study, volcanic glass fragments (n = 25) from Brothers volcano were analysed for their major element composition (Haase et al. 2006). The same samples were used in this study to investigate the chemistry of melt inclusions (n = 157), plagioclase crystals (n = 36), Fe–Ti oxides (n = 241) and magmatic sulphides (n = 134) by electron probe microanalysis using a JEOL JXA-8200 Superprobe at the GeoZentrum Nordbayern.
For the quantitative analyses of melt inclusions and their plagioclase host crystals an acceleration voltage of 15 kV and a beam current of 15 nA were used. A defocused beam of 10 and 3 µm, respectively, has been applied for these measurements to minimise the Na loss.
The natural glass standards VG-2 (basalt glass), VG-A99 (basalt glass) and VG-568 (rhyolite glass) of the Smithsonian Institution were analysed as secondary standards to monitor the precision an accuracy of the electron microprobe set up for the melt inclusion analyses (electronic supplement, Table A1). The same measurement conditions were used with the exception of a focused beam for the Fe–Ti oxide analyses. The FeO and Fe2O3 contents in Fe–Ti oxide were calculated based on the method presented by Lepage (2003). The analysed elements and the assigned standards are listed in Table A2 (electronic supplement). Different measurement conditions were applied for the analyses of the magmatic sulphides. Due to high concentrations of chalcophile elements, such as Cu and Ni with atomic numbers > 26 (Fe) a focused beam, an acceleration voltage of 20 kV and a beam current of 20 nA was used. The electron microprobe was calibrated by the following standards: FeS2 (S), Fe2O3 (Fe), CuFeS2 (Cu), NiO (Ni), Co (Co) and CaSi2O6 (Si). The count times were set to 20 and 10 s for the peak and background measurements, respectively.
The presented dataset was supplemented by previously published magmatic sulphide data from Brothers volcano (n = 128; Keith et al. 2017) and from mid-ocean ridges of the Atlantic (Kanehira et al. 1973; MacLean 1977; Mathez 1980; Petersen 1984; Patten et al. 2012, 2013; Marchesi et al. 2013), Pacific (Mathez and Yeats 1976; Foder et al. 1980; Distler et al. 1983; Schrader and Stow 1983; Francis 1990; Ackermand et al. 2007; Patten et al. 2012) and Indian Ocean (Francis 1990; Yang et al. 2014).
Laser Ablation ICP-MS
The Laser Ablation ICP-MS study was carried out at the GeoZentrum Nordbayern to determine the trace element composition of the volcanic glass fragments from Brothers volcano that have previously been studied by Haase et al. (2006) for their major element composition. For this purpose a UP193FX (New Wave Research) laser attached to an Agilent 7500i ICP-MS was used. The Laser operated with a frequency of 20 Hz, an irradiance of 0.52 GW/cm2 and a fluence of 2.5 J/cm2. The total analysis time for each spot was 50 s, including 20 s for gas blank analysis. The beam diameter was typically set to 50 µm and on occasion due to high amounts of phenocryst phases to 25 µm. The glass reference material NIST SRM 612 (Pearce et al. 1997) was used for the external calibration of Cu, Mo, La, Ce, Sm and Pb. Analytical precision and accuracy was monitored based on the repeated analyses of BCR-2 g and NIST SRM 614, as secondary standards, yielding < 4.2% RSD for all elements (electronic supplement, Table A3). To monitor the instrument drift the silicate standards were analysed several times during an analytical day. The trace element concentrations were calculated with Glitter (van Achterbergh et al. 2000) using Si as internal standard, determined by electron probe microanalyses as reported in Haase et al. (2006). Based on a single spot ablation pattern each glass sample was analysed at least 3 times for homogenous laser signals and up to 6 times for more heterogeneous compositions. Mean concentrations and standard deviations were calculated for each element based on multiple analyses in the different glass samples (electronic supplement, Table A4).
Results
Petrography
The petrographic observations reveal that globules of magmatic sulphides in the Brothers arc lavas are common but highly variable in size (< 10–300 µm). The round shape of most globules (Fig. 2), that are hosted in volcanic glass from the seafloor, suggest that they were not affected by metal leaching or fluid alteration to significant amounts indicating that they preserve the original composition of the former sulphide liquid (Mathez 1980). Sulphide globules are typically hosted in volcanic glass and occur in association with different silicate and oxide phases (Fig. 2). For instance, magmatic sulphide inclusions were observed in plagioclase (Fig. 2a) and clinopyroxene phenocrysts (Fig. 2b). However, they are most abundant in association with Fe–Ti oxides, which either host the globules (Fig. 2c, d) or served as nucleation sites during sulphide segregation indicated by a flat surface in contact to the oxide phase (Fig. 2c–e). Two types of Fe–Ti oxides were distinguished in reflected light: ilmenite and magnetite/ulvöspinel solid solutions; the two phases commonly show textural equilibrium (Fig. 2e).
Photomicrographs of samples from Brothers volcano in reflected light: a magmatic sulphide inclusion in plagioclase (SO135-66DR-01), b matrix- and clinopyroxene-hosted magmatic sulphides (SO135-59DR-01), c magmatic sulphides associated with Fe–Ti oxide (SO135-59DR-01; Keith et al. 2017), d Fe–Ti oxide phase acting as nucleation site during magmatic sulphide segregation (SO135-52DR-01), e ilmenite and ulvöspinel in textural equilibrium (SO135-52DR-01), f sulphides along a vesicle margin (SO135-66DR-01). cpx clinopyroxene, ilm ilmenite, plag plagioclase (figures in colour can be found in the web version of the article)
Pyrrhotite has been identified as the most abundant phase in the sulphide segregates from Brothers volcano (Fig. 2). Copper-rich sulphides (e.g., cubanite and chalcopyrite) were less common and Ni-rich phases are absent or must be sub-microscopic in size. This finding is in agreement with previous results showing that Ni-rich phases, such as pentlandite are rare or absent in island arc magmatic systems (Keith et al. 2017). Hence, most globules show a homogenous texture exclusively composed of pyrrhotite (n = 67), whereas heterogeneous globules (n = 19) and zoned segregates, in which distinct phases can be distinguished (n = 9) are rare. In addition, sulphides have been identified along vesicle margins (Fig. 2f), as described in previous studies from other localities (Francis 1990; Ackermand et al. 2007). Systematic mineralogical variations in sulphide globule occurrence between samples from the NW caldera and the cone complex of Brothers volcano were not observed.
Plagioclase and Fe–Ti oxide mineral chemistry
Anorthite contents in plagioclase vary over a small compositional range (An49–56, electronic supplement, Table A5) between andesine and labradorite. Differences between plagioclase cores (An49–55) and rims (An49–56) were not observed. Iron and Ti contents in the oxide phases vary between ilmenite (FeTiO3) and magnetite (Fe3O4). Oxides with compositions between these two potential endmembers can be classified as ulvöspinel (TiFe2O4, electronic supplement, Table A6).
Sulphide composition
Copper varies significantly in the Brothers magmatic sulphides from trace amounts in pyrrhotite to 24.1 wt% in cubanite. However, most sulphide segregates are characterised by Cu concentrations < 2 wt% (n = 225) and only a small proportion shows Cu contents > 2 wt% (n = 37, Fig. 3). Similarly, Ni-rich sulphides were not identified in lavas from Brothers volcano suggesting that pentlandite is at best a trace component in the sulphide globules (Fig. 3). Hence, the sulphide segregates from Brothers volcano are depleted in Ni compared to those hosted in basalts from mid-ocean ridges (Fig. 3). These results confirm the textural classification based on the petrographic observations. The dominance of compositions near stoichiometric pyrrhotite or cubanite is indicative for homogenous (consisting of one single phase) or zoned globules. Systematic variations in magmatic sulphide chemistry with respect to the host phase were not observed. Compositions between two stoichiometric endmembers, such as cubanite and pyrrhotite, are rare implying that fine-grained heterogeneously textured sulphides, where single phases cannot be analysed due to an overlapping electron beam (Keith et al. 2017), are uncommon at Brothers volcano.
Composition of magmatic sulphides from Brothers volcano in the Cu–Fe–Ni system, concentrations in at%. Orange symbols show the stoichiometric composition of bornite (bn), chalcopyrite (cpy), cubanite (cub), pentlandite (pent) and pyrrhotite (po). The Brothers data set includes the published analyses by Keith et al. (2017). Grey symbols represent literature data from mid-ocean ridges, as described in the text (cf. “Methods”). New data presented in this figure can be found in the electronic supplement (Table A7) (figures in colour can be found in the web version of the article)
Most sulphides (n = 40) that are attached to vesicle margins in glass samples from Brothers volcano were classified as pyrite based on their chemical composition. Only one analyses showed high Cu contents (22.8 wt%) in the range of cubanite (electronic supplement, Table A8).
Lava composition
The composition of volcanic glass samples revealed that the Brothers lavas vary in terms of their SiO2 contents over a small range from 66.3 to 72.4 wt%, i. e. from dacites to rhyolites. The glass samples from the volcanic cone are slightly more evolved than those from the caldera (Fig. 4; Haase et al. 2006). Published whole rock data shows SiO2 concentrations between 62.2 and 66.2 wt% (Wright and Gamble 1999; Smith et al. 2003; Timm et al. 2012), which are slightly lower than those of the volcanic glass samples (Fig. 4). Only one mafic glass sample of basaltic andesitic composition (SiO2 = 55.0 wt%, Fig. 4) was recovered from Brothers Ridge, which was interpreted as a young dyke-like structure beneath Brothers caldera (Wysoczanski et al. 2012).
Major element variation diagrams of arc and back-arc lavas. a MgO, b Al2O3, c TiO2, d FeOT, e K2O and f CaO vs. SiO2. The most primitive lava composition (SiO2 = 51.6 wt%; Turner et al. 1997) from L’Esperance volcano was used to perform the MELTS simulations. The different lines represent the isobaric (~ 0.71 kbar) MELTS simulations based on different starting parameters: solid (H2O = 0.5 wt%), dashed (H2O = 1.5 wt%), black (FMQ −1), dark grey (FMQ), light grey (FMQ +1). Data taken from literature as specified in the text (cf. Results, Lava composition). All analyses represent glass data if not specified otherwise. wr whole rock (figures in colour can be found in the web version of the article)
Due to the limited compositional range of the Brothers lavas the dataset was supplemented by melt inclusion, volcanic glass and whole rock data from other subduction zone-related magmatic systems to investigate the evolution of island arc magmas. This includes data from the L’Esperance (Ewart et al. 1994, 1998; Turner et al. 1997) and the Rumble III and IV (Wysoczanski et al. 2006, 2012) island arc volcanoes, as well as from the Valu Fa Ridge (Davis et al. 1990; Vallier et al. 1991; Peate et al. 2001; Haase et al. 2002; Fretzdorff et al. 2006; Jenner et al. 2015) and the Pual Ridge (Kamenetsky et al. 2001; Moss and Scott 2001; Yang and Scott 2002, 2005; Sun et al. 2004; Jenner et al. 2010, 2012), as examples for back-arc volcanism. Three groups of major elements can be distinguished based on their behaviour during magma evolution, i.e., with increasing SiO2, including elements with an (1) incompatible (K2O, Na2O), (2) compatible (MgO, Al2O3, CaO) and (3) an initially incompatible and later compatible (TiO2, FeOT) character (Fig. 4).
Volatile elements, such as Cl and S, show distinct variations in the Brothers melts (Fig. 5). Sulphur varies between trace and minor element levels from about 10 to 420 ppm possibly with a tendency to lower concentrations in the most evolved samples (Haase et al. 2006). In contrast, Cl is consistently high in all Brothers volcano glass samples with concentrations from 4370 to 6570 ppm. The S and Cl contents of plagioclase-hosted melt inclusions tend to be higher compared to those in the glass samples (Fig. 5).
Variation diagrams of a S and b Cl vs. SiO2. Sulphur and Cl variations at a given SiO2 concentration are interpreted to be due to magma degassing. Open symbols generally represent melt inclusion data of the glass and whole rock samples with the same colour coding. The grey field represents the glass composition of the Valu Fa Ridge lavas. The grey solid line displays the S concentration at sulphide saturation (SCSS) calculated based on the method presented by Smyth et al. (2017). Input parameters include the composition of the Brothers volcano sulphide droplets and the corresponding glass samples, as well as physical values, such temperature and pressure, as specified in the text. Data taken from literature as specified in the text (cf. “Results”, Lava composition). New data presented in this figure can be found in the electronic supplement (Table A9) (figures in colour can be found in the web version of the article)
Incompatible trace element ratios, such as La/Sm and Ce/Pb in the Brothers lavas (2.7 and 4.1, respectively) are similar to other island arc and back-arc systems showing average values of about 2.3 and 4.6, respectively. Only the Valu Fa Ridge data set (grey field) differs significantly due to lower La/Sm (av. 1.3) and higher Ce/Pb ratios (av. 9.0, Fig. 6).
Trace element ratios of a La/Sm and b Ce/Pb vs. SiO2. The grey field represents the glass composition of the Valu Fa Ridge lavas. Note the similar trace element ratios between Brothers volcano, Pual Ridge and the other island arc volcanoes suggesting a similar parental magma composition. Data taken from literature as specified in the text (cf. “Results”, Lava composition). New data presented in this figure can be found in the electronic supplement (Table A4) (figures in colour can be found in the web version of the article)
The transition metals Cu and Mo represent trace elements in the samples investigated in this study (Fig. 7). Copper either represents a compatible element (Valu Fa Ridge; Francis 1990) or shows a strong incompatible behaviour during the early stages of magma evolution (SiO2 < 60 wt%) followed by a sudden drop in Cu at about 60 wt% SiO2 and a slightly compatible behaviour in the more evolved melts (SiO2 > 60 wt%, Fig. 7a). In contrast, Mo contents increase with increasing SiO2 (Kamenetsky and Eggins 2012), however, variations in the slope of the linear trends with respect to the tectonic setting suggest that Mo is more incompatible in island arc than in back-arc magmas (Fig. 7b).
Variation diagrams of a Cu and b Mo vs. SiO2. The grey field represents the glass composition of the Valu Fa Ridge lavas. The black arrows represent the best fit regression of the, island arc (upper line), Pual Ridge (middle line) and Valu Fa Ridge (lower line) data set. The antithetic behaviour of Cu and Mo with proceeding magmatic differentiation lead to low Cu and high Mo contents in the evolved Brothers melts. Note the higher incompatibility of Mo in island arc compared to back-arc systems. Data taken from literature as specified in the text (cf. “Results”, Lava composition). New data presented in this figure can be found in the electronic supplement (Table A4) (figures in colour can be found in the web version of the article)
Discussion
Parental mafic analogue to the Brothers dacites
Ratios of incompatible trace elements with similar solid–liquid distribution coefficients show no variation during magmatic differentiation, and can therefore, be used to identify the source or mafic parental magma composition of a more evolved melt (Miller et al. 1994; Haase et al. 2006). The La/Sm and Ce/Pb ratios in samples from Brothers Ridge, L’Esperance, Rumble III and IV and Pual Ridge are similar to those from Brothers volcano indicating that mafic compositions from these localities may act as a parental magma analogue (Fig. 6). This conclusion is supported by Haase et al. (2006) showing that the basaltic L’Esperance and Rumble III lavas can be used as parental magmas to the evolved Brothers melts based on similar Ba/La as well as comparable Sr and Nd isotope ratios. Moreover, the L’Esperance, Rumble III and Brothers volcanoes are part of the same segment of the Kermadec arc (Ewart and Hawkesworth 1987; Haase et al. 2006). In contrast, the Valu Fa Ridge lavas vary in terms of their incompatible trace element ratios probably due to a different source or parental melt composition. Miller et al. (1994) and Beaudoin et al. (2007) have shown that the Ce/Pb ratio can be used to track the fluid contribution from the subducted slab to the mantle wedge, the source region for arc (and back-arc) magmas. Subduction zone fluids are enriched in Pb leading to low Ce/Pb ratios in systems with a strong subduction influence (e.g., Brothers volcano) compared to those, which are less affected by a subduction component (Valu Fa Ridge, Fig. 6b).
The thermodynamic modelling software Rhyolite-MELTS (Gualda et al. 2012) was used to calculate a liquid line of descent for the evolved Brothers lavas based on the assumption that the L’Esperance island basalts represent the parental magma of the Brothers dacites and rhyolites (Fig. 4). The MELTS modelling was conducted based on the most primitive lava composition (SiO2 = 51.6 wt%; Turner et al. 1997) from L’Esperance volcano assuming a uniform magma chamber pressure governed by a water depth of 1500 mbsl (~ 0.15 kbar) and a crustal depth of 2500 mbsf (~ 0.71 kbar; Gruen et al. 2014) yielding a total pressure of ~ 0.86 kbar. Variable redox conditions (FMQ −1, FMQ, FMQ +1) and H2O contents (0.5, 1.5 wt%) were applied for the calculations. The results are based on 10 °C temperature increments (Fig. 4). Fractionated phases include plagioclase (46–47%), clinopyroxene (33–44%), spinel (~ 7%) and apatite (~ 0.01%) matching the observed phenocrysts in the examined Brothers volcano samples (Fig. 2; Gamble and Wright 1995; Haase et al. 2002, 2006; de Ronde et al. 2005). The modelling curves resemble the observed chemical trends of the Brothers volcano dacites and rhyolites (Fig. 4), which confirms that the L’Esperance basalts can be used as a mafic parental melt to the Brothers lavas. Furthermore, the composition of the cone and the caldera lavas can be simulated by the same parental magma composition, which coincides with the identical incompatible trace element ratios and isotope compositions (Fig. 6; Haase et al. 2006). This is also in agreement with the similar chemical and mineralogical composition of the magmatic sulphides from the cone and the caldera of Brothers volcano (Fig. 3).
Thermodynamic investigations on the Brothers magmatic systems
Previous studies have shown that it is important to define the physicochemical conditions (T, p, fO2, H2O) in a fractionating magma because they control the S solubility limit in a silicate melt and therefore the behaviour of chalcophile metals and the composition of potential immiscible sulphide liquids (Distler et al. 1983; Mavrogenes and O’Neill 1999; Ackermand et al. 2007; Yang et al. 2014).
To determine the temperature and fO2 of the Brothers melts the magnetite-ulvöspinel geo-thermometer and oxygen barometer by Anderson and Lindsley (1985), integrated in the ILMAT spreadsheet (Lepage 2003) was used (electronic supplement, Table A6). The petrographic observations imply that the Fe–Ti oxides crystallised under equilibrium conditions (Fig. 2e). The calculated temperatures vary from 871 to 988 °C with an average value of 913 ± 14 °C (n = 248). Temperature variations between samples from the caldera (879–985 °C, n = 99) and the cone (871–988 °C, n = 149) of Brothers volcano were not observed. Similarly, the fO2 calculations revealed no systematic differences between these two sites, which show an overall variation from − 10.1 to − 11.9 log fO2 units, approximately FMQ − 0.6 to + 1.5 at 913 °C and ~ 0.86 kbar total pressure (Frost 1991). The fO2 estimations for Brothers volcano are comparable to other island arc systems that typically range from FMQ 0 to FMQ +2 (Parkinson and Arculus 1999; Lee et al. 2012; Wysoczanski et al. 2012). The fO2 of the Brothers magmas is also comparable to the Lau back-arc (Nilson and Peach 1993) explaining similar fractionation processes in the magmas (Fig. 4). At FMQ −1 to FMQ 0 the dominant S species is S2−, whereas at FMQ above +1 most S exists as S6+ and between FMQ 0 and FMQ +1 both S2− and S6+ are stable (Jugo et al. 2010). This indicates that in the Brothers melts S most likely exists as S2− and S6+. The high concentrations of S in the Brothers melts (up to 740 ppm) reaching the sulphide saturation limit (Fig. 5a) explain the high abundance of magmatic sulphides in the Brothers volcano samples (Fig. 2), which also coincides with the existence of S2−. The melt inclusions tend to plot above the calculated sulphide saturation limit (Fig. 5a), which may be due to the analyses of melt inclusion-hosted invisible sulphide segregates by the defocused beam (10 µm) of the electron microprobe.
The H2O contents of the Brothers volcano melts were estimated based on the plagioclase-melt hygro-thermometer (electronic supplement, Table A5) by Putirka (2008) and Lange et al. (2009). Crystallisation temperatures varied between 987 and 1034 °C with an average error of ± 38 °C (Putirka 2008). Lower temperatures were generally observed for plagioclase rims (987–993 °C) compared to the corresponding cores (1021–1034 °C), which are also above the temperatures determined by magnetite-ulvöspinel geo-thermometer (871–988 °C). This is in agreement with the onset of the plagioclase crystallisation prior to Fe–Ti oxides in tholeiitic magmatic systems (Fig. 4; Haase et al. 2006). Estimated H2O contents varied between 2.3 and 4.1 wt%, which is comparable to previous suggestions (H2O = 2–3 wt%) for the Brothers dacites (Haase et al. 2006). Calculations based on the composition of plagioclase rims generally yielded higher H2O contents (3.4 to 4.1 wt%) compared to the cores of the same crystal (2.3–2.8 wt%) reflecting the typical incompatible behaviour of H2O during fractional crystallisation (Schmitt 2001).
The H2O saturation limit of the Brothers melts was calculated in an isobaric system (0.86 kbar) using the empirical model of Moore et al. (1998). Compositional variations with respect to the lava chemistry of Brothers volcano (SiO2 = 66.5–72.4 wt%) had no effect on the saturation limit. However, temperature changes significantly influenced the H2O saturation limit. Based on the temperature estimations the H2O solubility limit was calculated between 850 and 1050 °C yielding a maximum H2O content of 3.7 and 3.2 wt%, respectively. This suggests that the Brothers melts reached the H2O saturation limit during crystallisation (between 66.4 and 77.4 wt% SiO2) and storage at 0.86 kbar (electronic supplement, Fig. A1). Importantly, the temperature, fO2 and H2O estimations between the thermodynamic calculations and the MELTS simulations are comparable, which confirms that the presented results are in a reliable range (electronic supplement, Fig. A1).
Formation of magmatic sulphides and their potential role as a Cu source
The petrographic results imply that sulphide–silicate melt immiscibility represents a continuous or multistage process in the Brothers volcanic system (cf. Keith et al. 2017). This conclusion is based on the sulphide–silicate mineral relationships indicating that sulphide segregation occurs during different stages of magmatic differentiation in association with plagioclase, clinopyroxene and in particular Fe–Ti oxide fractionation (Fig. 2). The S concentration at sulphide saturation in silicate melts is controlled by temperature, pressure, fO2, the degree of fractional crystallisation and the initial S and Fe contents of the magma (Mathez and Yeats 1976; Distler et al. 1983; Mavrogenes and O’Neill 1999; Ackermand et al. 2007; Yang et al. 2014). However, fO2 seems to be by far the most important parameter controlling the solubility of S (Jenner et al. 2010; Jugo et al. 2010; Keith et al. 2017). Magmatic sulphides that post-date the Fe–Ti oxides (Fig. 2c–e) and the occurrence of S2− and S6+ in the Brothers melts suggest that the formation of these late stage sulphides may be related to the proposed “magnetite crisis”, the reduction of S6+ to S2− caused by fO2 changes in the Fe–Ti oxide fractionating magma (Jenner et al. 2010).
Recent studies have shown that magmatic sulphides represent an important source of chalcophile metals in hydrothermal fluids and associated sulphide precipitates (Patten et al. 2016a, b). Arc-hosted hydrothermal systems are known to be economically important due to their high Cu contents (Sillitoe 2010; Richards 2011). Copper is highly compatible in immiscible sulphide liquids as indicated by high sulphide liquid-silicate melt partition coefficients between 600 and 1500 (Peach et al. 1990; Lynton et al. 1993; Gaetani and Grove 1997; Ripley et al. 2002; Li and Audétat 2012; Patten et al. 2013; Li 2014). Hence, Cu will strongly partition into a segregating sulphide phase leaving behind a Cu depleted silicate melt. This coincides with the observed Cu depletion in the Brothers melts (Fig. 4) and the Cu enrichment (up to 24.1 wt%, Fig. 3) in the magmatic sulphides hosted in the Brothers lavas. This can likely be explained by the proposed “magnetite crisis” (Jenner et al. 2010) causing sulphide saturation in association Fe–Ti-oxide fractionation (Fig. 2c–e). The occurrence of Cu-rich sulphides (Fig. 3) further imply that wall rock leaching in the reaction and fluid upflow zone represents a likely process that can explain the high Cu concentrations (up to 35.6 wt%) in the seafloor massive sulphide ores (de Ronde et al. 2011). However, previous studies also suggested that Cu and other chalcophile metals are contributed to the Brothers hydrothermal system by exsolving magmatic volatiles from the underlying magma chamber (de Ronde et al. 2011; Keith et al. 2016a), possibly suggesting a bimodal Cu source.
Evidence for Cu degassing in the Brothers magmatic system
Arc magmas commonly reach volatile saturation during differentiation in the upper crust (Wallace 2005). Vapour bubbles in plagioclase-hosted melt inclusions from Brothers volcano (electronic supplement, Fig. A2) indicate that the magma was saturated with volatiles in the magma chamber suggesting pre-eruptive degassing of an immiscible fluid phase (Yang and Scott 1996, 2002). Those melt inclusions are enriched in S (and Cl) compared to the corresponding glass matrix (Fig. 5; electronic supplement, Fig. A3). The presented thermodynamic calculations suggest that the Brothers volcano melts reached H2O saturation between 3.2 and 3.7 wt% H2O during their evolution and that both S2− and S6+ were present. Hence, an aqueous S- and Cl-rich magmatic volatile phase with variable proportions of SO2 and H2S probably exsolved from the Brothers volcano melts. Copper can form stable complexes with reduced S but also shows a strong affinity to Cl in magmatic volatiles (Williams-Jones and Heinrich 2005; Pokrovski et al. 2008; Zajacz and Halter 2009; Zajacz et al. 2011; Blundy et al. 2015). Although high Cu solubility in a magmatic aqueous vapour phase is under discussion (Lerchbaumer and Audétat 2012), experimental studies have shown that mixed ligand complexes including S and Cl are the most stable Cu complexes (e.g., CuCl(HS)−) representing the most efficient form to transport Cu in magmatic volatiles (Simon et al. 2006; Mei et al. 2013). Such a magmatic volatile phase can contribute Cu and other chalcophile metals to an overlying hydrothermal system, which can result in high concentrations of these metals in the seafloor massive sulphides (de Ronde et al. 2005; Berkenbosch et al. 2012; Gruen et al. 2014).
It is likely that this model is applicable for the Brothers volcano magmatic-hydrothermal system. For example, negative S isotope values to − 4.7‰ in hydrothermal sulphides from the Brothers vents were interpreted to be the result of an isotopically light magmatic volatile phase, which was added to the hydrothermal system by sulphur disproportionation (de Ronde et al. 2011). The plagioclase-hosted melt inclusions from Brothers volcano show Cu concentrations of up to 69 ppm, whereas Cu in the corresponding glass matrix is generally below 21 ppm indicating Cu loss (electronic supplement, Fig. A4; de Ronde et al. 2011). Importantly, the Cu enrichment in the melt inclusions cannot be explained by Cu diffusion from the host mineral, since Cu is highly incompatible in plagioclase and other silicates (Jenner et al. 2010; de Ronde et al. 2011). Sulphide occurrences along vesicle margins (Fig. 2F) emphasise that a S-rich volatile phase exsolved from the magma (Francis 1990; Ackermand et al. 2007; Blundy et al. 2015). Alternatively, they could represent sulphide compounds transported by floatation of sulphide melt on vapour bubbles (Mungall et al. 2015), which could also explain the proposed occurrence of melt inclusion-hosted sulphide droplets (Fig. 5a). These sulphides almost exclusively consists of pyrite (n = 40), only one Cu-rich phase with a composition close to cubanite was identified (electronic supplement, Table A8). Iron diffusion from the melt to the vesicle margin where it reacted with S-bearing volatiles possibly explains the precipitation of pyrite (Ackermand et al. 2007). In contrast, the low Cu contents in the Brothers volcano melts (Fig. 7a) suggest that diffusion cannot explain the occurrence of cubanite. Hence, it is more likely that Cu was transported in an exsolving volatile phase together with S and possibly Cl (Fig. 5; Francis 1990; Blundy et al. 2015). The rare occurrence of Cu-sulphides along the vesicle margins may imply that most of the degassed Cu did not precipitate but rather was transported in the volatile phase to shallower crustal levels where it interacted with the circulating hydrothermal fluids. Therefore, it is likely that a Cu-bearing magmatic volatile phase exsolved from the Brothers melts, which added Cu to the overlying hydrothermal system in addition to the common leaching process of Cu from the wall rock-hosted magmatic sulphides (Fig. 3).
The Brothers magmatic-hydrothermal system: A submarine analogue for subaerial epithermal–porphyry deposits?
It was shown that H2O-rich arc magmas in the upper crust have the potential to exsolve magmatic volatiles that can carry significant amounts of chalcophile metals to form a magmatic-hydrothermal deposit (e.g., Richards 2011). Advanced argillic alteration assemblages, enargite-bearing veins and elemental S occur at Brothers volcano indicating high-sulphidation conditions similar to subaerial epithermal–porphyry systems (de Ronde et al. 2005). The dacites and rhyolites that host the Brothers volcano magmatic-hydrothermal system are highly depleted in Cu (Fig. 7a) suggesting that Cu was extracted from the silicate melt during the segregation of an immiscible sulphide liquid preserved as wall rock-hosted magmatic sulphides representing a potential Cu source for hydrothermal fluids. In addition, the results presented here suggest that a Cu-rich volatile phase exsolved from the Brothers melts, which also contributed Cu to the overlying hydrothermal system resulting in the precipitation of Cu-rich massive sulphides at the seafloor (up to 35.6 wt%; de Ronde et al. 2011).
Copper and Mo show a compatible and incompatible behaviour during magmatic differentiation leading to low Cu and high Mo contents (Fig. 7), as well as low Cu/Mo ratios in the evolved Brothers melts (Fig. 8). Importantly, Cu has the stronger affinity to partition into an exsolving magmatic volatile phase as shown by volatile-silicate melt partition coefficients between 10 and 100 (Candela and Holland 1984; Zajacz et al. 2008; Guo and Audétat 2017). In contrast, the volatile-silicate melt partition coefficients for Mo is between 0.1 and 4 (Candela and Holland 1984; Guo and Audétat 2017). Consequently, associated magmatic volatiles will characteristically be enriched in Cu compared to Mo. leading to a high Cu/Mo ratio. This chemical signature may be preserved in associated sulphide precipitates from hydrothermal systems that are affected by a magmatic volatile component (Fig. 8).
Diagram of Cu/Mo vs. Cu. Symbols in grey scale generally represent the bulk ore composition of the submarine massive sulphides, whereas coloured symbols show the composition of the associated magmatic host rocks. Data from subaerial high-sulphidation epithermal–porphyry system were added for reference including the bulk ores (white field) and the least altered host rock compositions of these systems (grey field). Ore samples from the selected arcs generally show higher Cu and Cu/Mo ratios than there associated host rocks, which are interpreted to be the result of a Cu-rich and Mo-poor magmatic volatile phase added to the hydrothermal system. Data for the submarine systems taken from literature as specified in the text (cf. “Results”, Lava composition). The OSNACA data base (http://www.cet.edu.au/) and the references listed in the electronic supplement (A1) have been used as the data source for the subaerial systems. New data presented in this figure can be found in the electronic supplement (Table A4). All analyses from the submarine systems represent glass data if not specified otherwise. VFR Valu Fa Ridge, wr whole rock (figures in colour can be found in the web version of the article)
Data from high-sulphidation epithermal and porphyry Cu deposits (grey and white field) were compared with the Brothers magmatic-hydrothermal system to test this hypothesis (Fig. 8). Magmatic volatiles are the main source and transport medium for Cu in these subaerial systems and their Cu concentrations and Cu/Mo ratios in the bulk ores and in least altered host rock samples match those from Brothers volcano remarkably well (Fig. 8). This implies for Brothers volcano that Cu was also added to the hydrothermal system by magmatic volatiles and this signature is preserved in the seafloor sulphide precipitates. Importantly, this seems to be no local feature because similar Cu concentrations and Cu/Mo ratios in magmatic rocks and bulk ore samples were observed for other island arc and back-arc systems (Fig. 8). Therefore, we conclude that magmatic volatiles represent an important source for Cu in addition to wall rock leaching in the reaction and fluid upflow zone of submarine magmatic-hydrothermal arc and back-arc systems.
Summary and conclusions
The results presented here imply that a bimodal Cu contribution to the Brothers volcano magmatic-hydrothermal system can best explain the occurrence of the Cu-rich seafloor massive sulphide ores (up to 35.6 wt%). High Cu contents (up to 24.1 wt%) in the magmatic sulphides from Brothers volcano suggest that wall rock leaching of such sulphides in the reaction and fluid upflow zone represents a likely source of Cu for the hydrothermal system and its precipitates. In addition, the combined use of chemical, mineralogical and thermodynamic investigations revealed that the Brothers melts reached volatile saturation during their evolution. Hence, an aqueous S- and Cl-rich magmatic volatile phase extracted some of the Cu from the silicate melt. This Cu-rich volatile phase ascended to shallower crustal levels where it contributed Cu to the hydrothermal fluids from which the Cu-rich seafloor massive sulphides formed.
High Cu/Mo ratios as suggested for the magmatic volatiles are preserved in the seafloor sulphide precipitates, which are probably due to the distinct behaviour of Cu and Mo during magmatic differentiation and volatile exsolution. Similar Cu/Mo ratios were observed in subaerial high-sulphidation epithermal–porphyry systems, where magmatic volatiles represent the main source for Cu. Therefore, we conclude that shallow marine arc (and back-arc) magmatic-hydrothermal-systems share mineralogical and chemical features with epithermal–porphyry deposits on land suggesting that these active systems represent a new hybrid seafloor analogue between those subaerial systems and classic Cyprus-type VHMS deposits. Importantly, our results further suggest that this is no local feature since similar Cu/Mo ratios in magmatic rocks and bulk ore samples were observed for other island arc and back-arc systems. Hence, future investigations have to focus on the source characteristics and the enrichment processes of other chalcophile and economically critical trace metals (e.g., Au and Te) to identify new sites and to assess the potential of such locations for submarine mining operations.
References
Ackermand D, Hekinian R, Stoffers P (2007) Mineralogy of magmatic sulfides and transition metal oxides from lavas of the Pitcairn Hotspot and the Pacific Antarctic Ridge. N Jb Miner Abh 184/1:77–94
Anderson DJ, Lindsley DH (1985) New (and final!) models for the Ti-magnetite/ilmenite geothermometer and oxygen barometer. Eos Trans AGU 66:416
Baker ET, Walker SL, Embley RW, de Ronde CEJ (2012) High-resolution hydrothermal mapping of brothers caldera, Kermadec. Arc Econ Geol 107:1583–1593
Beaudoin Y, Scott SD, Gorton MP, Zajacz Z, Halter W (2007) Pb and other ore metals in modem seafloor tectonic environments: evidence from melt inclusions. Mar Geol 242:271–289
Berkenbosch HA, de Ronde CEJ, Gemmell JB, McNeill AW, Goemann K (2012) Mineralogy and formation of black smoker chimneys from brothers submarine volcano, Kermadec Arc. Econ Geol 107:1613–1633
Blundy J, Mavrogenes J, Tattitch B, Sparks S, Gilmer A (2015) Generation of porphyry copper deposits by gas-brine reaction in volcanic arcs. Nature 8:235–240
Candela PA, Holland HD (1984) The partitioning of copper and molybdenum between silicate melts and aqueous fluids. Geochim Cosmochim Acta 48:373–380
Caratori Tontini F, de Ronde CEJ, Yoerger D, Kinsey J, Tivey M (2012) 3-D focused inversion of near-seafloor magnetic data with application to the Brothers volcano hydrothermal system, Southern Pacific Ocean, New Zealand. J Geophys Res 117:1–12
Craddock PR, Bach W (2010) Insights to magmatic-hydrothermal processes in the manus back-arc basin as recorded by anhydrite. Geochim Cosmochim Acta 74:5514–5536
Craddock PR, Bach W, Seewald JS, Rouxel OJ, Reeves E, Tivey MK (2010) Rare earth element abundances in hydrothermal fluids from the manus basin, papua new guinea: indicators of sub-seafloor hydrothermal processes in Back-Arc basins. Geochim Cosmochim Acta 74:5494–5513
Davis AS, Clague DA, Morton JL (1990) Volcanic glass compositions from two spreading centers in Lau Basin, southwest Pacific Ocean. Geol Jahrb 92:481–501
de Ronde CEJ, Baker ET, Massoth GJ, Lupton JE, Wright I, Feely RA, Greene RR (2001) Intra-oceanic subduction-related hydrothermal venting, Kermadec volcanic arc, New Zealand. Earth Planet Sci Lett 193:359–369
de Ronde CEJ, Hannington MD, Stoffers P, Wright I, Ditchburn RG, Reyes AG, Baker ET, Massoth GJ, Lupton JE, Walker SL, Greene RR, Soong CWR, Ishibashi J, Lebon GT, Bray CJ, Resing JA (2005) Evolution of a submarine magmatic-hydrothermal system: Brothers volcano, southern Kermadec arc, New Zealand. Econ Geol 100:1097–1133
de Ronde CEJ, Massoth GJ, Butterfield DA, Christenson BW, Ishibashi J, Ditchburn RG, Hannington MD, Brathwaite RL, Lupton JE, Kamenetsky VS, Graham IJ, Zellmer GF, Dziak RP, Embley RW, Dekov VM, Munnik F, Lahr J, Evans LJ, Takai K (2011) Submarine hydrothermal activity and gold-rich mineralization at Brothers volcano, Kermadec Arc, New Zealand. Miner Deposita 46:541–584
de Ronde CEJ, Hein JR, Butterfield DA (2014) Metallogenesis and mineralization of intraoceanic arcs II: the Aeolian, Izu-Bonin, Mariana, and Kermadec Arcs, and the Manus Backarc Basin-Introduction. Econ Geol 109:2073–2077
Distler VV, Petersen NN, Boronikhin VA (1983) Sulfide petrology of basalts from Deep Sea Drilling Project, Holes 504B, 505B. Initial Rep Deep Sea Drill Proj 69:607–617
Einaudi MT, Hedenquist JW, Inan E (2003) Sulfidation state of fluids in active and extinct hydrothermal systems: transitions from porphyry to epithermal environments. Econ Geol 10:285–314
Embley RW, de Ronde CEJ, Merle SG, Davy B, Tontini C, F (2012) Detailed morphology and structure of an active submarine arc caldera: Brothers volcano, Kermadec arc. Econ Geol 107:1557–1570
Ewart A, Hawkesworth CJ (1987) The pleistocene recent tonga Kermadec arc lavas—interpretation of new isotopic and rare-earth data in terms of a depleted mantle source model. J Petrol 28:495–530
Ewart A, Byran WB, Chappell BW, Rudnick RL (1994) Regional geochemistry of the Lau-Tonga arc backarc system. In: Hawkins J, Parson L, Allan J et al (eds) Proceedings of the Ocean Drilling Program, Scientific Results, vol 135, pp 385–425
Ewart A, Collerson KD, Regelous M, Wendt JI, Niu YL (1998) Geochemical evolution within the Tonga-Kermadec Lau arc back-arc systems: the role of varying mantle wedge composition in space and time. J Petrol 39:331–368
Foder RV, Berkeley JL, Keil K, Husler JW, Ma MS, Schmitt RA (1980) Petrology of basalt drilled from the Galapogos spreading center. Initial Reports. Deep Sea Drill Proj 54:737–749
Fontboté L, Kouzmanov K, Chiaradia M, Pokrosvkoi GS (2017) Sulfide minerals in hydrothermal deposits. Elements 13:97–103
Fortin M-A, Riddle J, Desjardins-Langlais Y, Baker DR (2015) The effect of water on the sulfur concentration at sulfide saturation (SCSS) in natural melts. Geochim Cosmochim Acta 160:100–116
Francis RD (1990) Sulfide globules in mid-ocean ridge basalts (MORB), and the effect of oxygen abundance in Fe–S–O liquids on the ability of those liquids to partition metals from MORB and komatiite magmas. Chem Geol 85:199–213
Fretzdorff S, Schwarz-Schampera U, Gibson HL, Garbe-Schönberg C-D, Hauff F, Stoffers P (2006) Hydrothermal activity and magma genesis along a propagating back-arc basin: Valu Fa Ridge (southern Lau Basin). J Geopyhs Res 111:1–17
Frost BR (1991) Introduction to oxygen fugacity and its petrologic importance. Rev Min 25:1–9
Gaetani GA, Grove TL (1997) Partitioning of moderately siderophile elements among olivine, silicate melt, and sulfide melt: constraints on core formation in the Earth and Mars. Geochim Cosmochim Acta 61:1829–1846
Gamble JA, Wright IC (1995) The southern Havre Trough: geological structure and magma petrogenesis of an active backarc rift complex. In: Taylor B (ed) Backarc Basin. Tectonics and Magmatism, Plenum Press, New York, pp 29–62
Gena K (2013) Deep sea mining of submarine hydrothermal deposits and its possible environmental impact in Manus Basin, Papua New Guinea. Procedia Earth Planet Sci 6:226–233
Gruen G, Weis P, Driesner T, Heinrich CA, de Ronde CEJ (2014) Hydrodynamic modeling of magmatic–hydrothermal activity at submarine arc volcanoes, with implications for ore formation. Earth Planet Sci Lett 404:307–318
Gualda GAR, Ghiorso MS, Lemons RV, Carley TL (2012) Rhyolite-MELTS: a modified calibration of MELTS optimized for Silica-rich, Fluid-bearing magmatic systems. J Petrol 53:875–890
Guo HH, Audétat A (2017) Transfer of volatiles and metals from mafic to felsic magmas in composite magma chambers: an experimental study. Geochim Cosmochim Acta 198:360–378
Haase KM, Worthington TJ, Stoffers P, Garbe-Schönberg D (2002) Mantle dynamics, element recycling, and magma genesis beneath the Kermadec Arc-Havre Trough. Geochem Geophys Geosyst 3:1–22
Haase KM, Stroncik N, Garbe-Schönberg D, Stoffers P (2006) Formation of island arc dacite magmas by extreme crystal fractionation: An example from Brothers Seamount, Kermadec island arc (SW Pacific). J Volcanol Geoth Res 152:316–330
Hannington MD, Galley AG, Herzig PM, Petersen S (1998) Comparison of the TAG mound and stockwork complex with Cyprus-type massive sulfide deposits. In: Herzig PM, Humphris SE, Mueller DJ, Zierenberg RA (eds) Proceedings of the Ocean Drilling Program, Scientific Results, vol 158, pp 389–415
Hannington M, Jamieson J, Monecke T, Petersen S, Beaulieu S (2011) The abundance of seafloor massive sulfide deposits. Geology 39, 1155–1158
Hedenquist JW, Lowenstern JB (1994) The role of magmas in the formation of hydrothermal ore deposits Nature 370, 519–527
Hedenquist JW, Arribas A, Reynolds TJ (1998) Evolution of an intrusion-centered hydrothermal system; far Southeast-Lepanto porphyry and epithermal Cu–Au deposits, Philippines. Econ Geol 93:373–404
Jenner FE, O’Neilll HSC, Arculus RJ, Mavrogenes JA (2010) The magnetite crisis in the evolution of arc-related magmas and the initial concentration of Au, Ag and Cu. J Petrol 51:2445–2464
Jenner FE, Acrculus RJ, Mavrogenes JA, Dyriw NJ, Nebel O, Hauri EH (2012) Chalcophile element systematics in volcanic glasses from the northwestern Lau Basin. Geochem Geophys Geosyst 13:1–25
Jenner FE, Hauri EH, Bullock ES, Konig S, Arculus RJ, Mavrogenes JA, Mikkelson N, Goddard C (2015) The competing effects of sulfide saturation versus degassing on the behavior of the chalcophile elements during the differentiation of hydrous melts. Geochem Geophy Geosy 16:1490–1507
Jugo PJ, Wilke M, Botcharnikov RE (2010) Sulfur K-edge XANES analysis of natural and synthetic basaltic glasses: implications for S speciation and S content as function of oxygen fugacity. Geochim Cosmochim Acta 74:5926–5938
Kamenetsky VS, Eggins SM (2012) Systematics of metals, metalloids, and volatiles in MORB melts: effects of partial melting, crystal fractionation and degassing (a case study of Macquarie Island glasses). Chem Geol 302–303:76–86
Kamenetsky VS, Binns RA, Gemmell JB, Crawford AJ, Mernagh TP, Maas R, Steele D (2001) Parental basaltic melts and fluids in eastern Manus backarc Basin: implications for hydrothermal mineralisation. Earth Planet Sci Lett 184:685–702
Kanehira K, Yui S, Sakai H, Sasaki A (1973) Sulphide globules and sulphur isotope ratios in the abyssal tholeiite from the Mid-Atlantic Ridge near 30°N latitude. Geochem J 7:89–96
Keith M, Häckel F, Haase KM, Schwarz-Schampera U, Klemd R (2016a) Trace element systematics of pyrite from submarine hydrothermal vents. Ore Geol Rev 72:728–745
Keith M, Haase KM, Klemd R, Krumm S, Strauss H (2016b) Systematic variations of trace element and sulfur isotope compositions in pyrite with stratigraphic depth in the Skouriotissa volcanic-hosted massive sulfide deposit, Troodos ophiolite, Cyprus. Chem Geol 423:7–18
Keith M, Haase KM, Klemd R, Schwarz-Schampera U, Franke H (2017) Systematic variations in magmatic sulphide chemistry from mid-ocean ridges, back-arc basins and island arcs. Chem Geol 451:67–77
Kelley KA, Cottrell E (2012) The influence of magmatic differentiation on the oxidation state of Fe in a basaltic arc magma. Earth Planet Sci Lett 3289–330:109–121
Kilias SP, Nomikou P, Papnikolaou D, Polymenakou PN, Godelitsas A, Argyraki A, Carey S, Gamaletsos P, Mertzimekis TJ, Christakis C, Croff Bell K, Soullos M (2013) New insights into hydrothermal vent processes in the unique shallow-submarine arc-volcano Kolumbo (Santorini), Greece. Sci Rep 3:1–13
Kilias SP, Gousgouni M, Godelitsas A, Gamaletsos P, Mertzimekis TJ, Nomikou P, Argyraki A, Goettlicher J, Steininger R, Papanikolaou, D (2016) Antimony fixation in solid phases at the hydrothermal field of Kolumbo submarine arc-volcano (Santorini): deposition model and environmental implications. Bulletin Geol Soc Greece L:2200–2209
Lange RA, Frey HM, Hector J (2009) A thermodynamic model for the plagioclase-liquid hygrometer/thermometer. Am Miner 94:494–506
Lee CA, Luffi P, Chin EJ, Bouchet R, Dasgupta R, Morton DM, Le Roux V, Yin Q, Jin D (2012) Copper systematics in Arc Magmas and implications for Crust-Mantle differentiation. Science 336:64–68
Lepage LD (2003) ILMAT: an excel worksheet for ilmenite–magnetite geothermometry and geobarometry. Comput Geosci 29:673–678
Lerchbaumer L, Audétat A (2012) High Cu concentrations in vapour-type fluid inclusions: an artifact ? Geochim Cosmochim Acta 88:255–274
Li Y (2014) Chalcophile element partitioning between sulfide phases and hydrous mantle melt: applications to mantle melting and the formation of ore deposits. J Asian Earth Sci 95:77–93
Li Y, Audétat A (2012) Partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb,W, Au, Pb, and Bi between sulfide phases and hydrous basanite melt at upper mantle conditions. Earth Planet Sci Lett 355–356:327–340
Lynton SJ, Candela PA, Piccoli PM (1993) An experimental study of the partitioning of copper between pyrrhotite and a high silica rhyolitic melt. Econ Geol 88:901–915
MacLean WH (1977) Sulfides in the core from leg 37 drill holes. Initial Rep Deep Sea Drill Proj 37:875–881
Marchesi C, Garrido CJ, Harvey J, Gonzalez-Jimenez JM, Hida K, Lorand J-P, Gervilla F (2013) Platinum-group elements, S, Se and Cu in highly depleted abyssal peridotites from the Mid-Atlantic Ocean Ridge (ODP Hole 1274A): Influence of hydrothermal and magmatic processes. Contrib Miner Petrol 166:1521–1538
Mathez EA (1980) Sulfide relations in Hole 814A flows and sulfur contents of glasses. Initial Rep Deep Sea Drill Proj 51:1069–1085
Mathez EA, Yeats RS (1976) Magmatic sulfides in basalt glass from dsdp hole 319a and site 320, Nazca plate. Initial Rep Deep Sea Drill Proj 34:363–373
Mavrogenes JA, O’Neill HSC (1999) The relative effects of pressure, temperature and oxygen fugacity on the solubility of sulfide in mafic magmas. Geochim Cosmochim Acta 63:1173–1180
Mei Y, Sherman DM, Liu WH, Brugger J (2013) Ab initio molecular dynamics simulation and free energy exploration of copper(I) complexation by chloride and bisulfide in hydrothermal fluids. Geochim Cosmochim Acta 102:45–64
Miller DM, Goldstein SL, Langmuir CH (1994) Cerium lead and lead-isotope ratios in arc magmas and the enrichment of lead in the continents. Nature 368:514–520
Moore G, Vennemann T, Carmichael ISE (1998) An empirical model for the solubility of H2O in magmas to 3 kilobars. Am Miner 83:36–42
Moss R, Scott RB (2001) Gold content of eastern Manus Basin volcanic rocks: implications for enrichment in associated hydrothermal precipitates. Econ Geol 96:91–107
Mungall JE, Brenan JM, Godel B, Barnes SJ, Gaillard F (2015) Transport of metals and sulphur in magmas by flotation of sulphide melt on vapour bubbles. Nature 8:216–219
Nilson K, Peach CL (1993) Sulfur speciation, oxidation state, and sulfur concentration in backarc magmas. Geochim Cosmochim Acta 57:3807–3813
Park JW, Campbell IH, Kim J, Moon JW (2015) The role of late sulfide saturation in the formation of a Cu- and Au-rich magma: insights from the platinum group element geochemistry of Niuatahi-Motutahi lavas, Tonga Rear Arc. J Petrol 56:59–81
Parkinson IJ, Arculus RJ (1999) The redox state of subduction zone: Insights from arc peridotites. Chem Geol 160:409–423
Patten C, Barnes SJ, Mathez EA (2012) Textural variations in MORB sulfide droplets due to differences in crystallization history. Can Miner 50:675–692
Patten C, Barnes SJ, Mathez EA, Jenner FE (2013) Partition coefficients of chalcophile elements between sulfide and silicate melts and the early crystallization history of sulfide liquid: LA–ICP–MS analysis of MORB sulfide droplets. Chem Geol 358:170–188
Patten CGC, Pitcairn IK, Teagle DAH, Harris M (2016a) Sulphide mineral evolution and metal mobility during alteration of the oceanic crust: Insights from ODP Hole 1256D. Geochim Cosmochim Acta 193:132–159
Patten CGC, Pitcairn IK, Teagle DAH, Harris M (2016b) Mobility of Au and related elements during the hydrothermal alteration of the oceanic crust: implications for the sources of metals in VMS deposits. Miner Deposita 51:179–200
Patten CGC, Pitcairn IK, Teagle DAH (2017) Hydrothermal mobilisation of Au and other metals in supra-subduction oceanic crust: Insights from the Troodos ophiolite. Ore Geol Rev 86:487–508
Peach CL, Mathez EA, Keays RR (1990) Sulfide melt-silicate melt distribution coefficients for noble metals and other chalcophile elements as deduced from MORB: implications for partial melting. Geochim Cosmochim Acta 54:3379–3389
Pearce NJG, Perkins WT, Westgate JA, Gorton MP, Jackson SE, Neal CR, Chenery SP (1997) A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostand Newslett 21:115–144
Peate DW, Kokfelt TF, Hawkesworth CJ, Van Calsteren PW, Hergt JM, Pearce JA (2001) U-series isotope data on Lau Basin glasses: the role of subduction-related fluids during melt generation in back-arc basins. J Petrol 42:1449–1470
Petersen N (1984) Ore mineralogy of South Atlantic basalts. Initial Rep Deep Sea Drilling Project 73:603–606
Pokrovski GS, Borisova AY, Harrichoury JC (2008) The effect of sulfur an vapor-liquid fractionation of metals in hydrothermal systems. Earth Planet Sci Lett 266:345–362
Putirka KD (2008) Thermometers and Barometers for Volcanic Systems. Rev Miner Geochem 69:61–120
Richards JP (2011) Magmatic to hydrothermal metal fluxes in convergent and collided margins. Ore Geol Rev 40:1–26
Ripley EM, Brophy JG, Li C (2002) Copper solubility in a basaltic melt and sulfide liquid/silicate melt partition coefficients of Cu and Fe. Geochim Cosmochim Acta 66:2791–2800
Schmitt AK (2001) Gas-saturated crystallization and degassing in large-volume, crystal-rich dacitic magmas from the Altiplano-Puna, northern Chile. J Geophys Res 106:30561–30578
Schrader EL, Stow SH (1983) Geochemistry and mineralogy of fresh and altered basalts from the Galapagos Rift. Initial Rep Deep Sea Drill Proj 70:391–401
Seedorff E, Dilles JH, Proffett JM, Einaudi MT, Zurcher L, Stavast WJA, Johnson DA, Barton MD (2005) Porphyry deposits: characteristics and origin of hypogene features. Econ Geol 100:251–298
Sillitoe RH (2010) Porphyry copper systems. Econ Geol 105:3–41
Simmons SF (2005) Geological characteristics of epithermal precious and base metal deposits. Econ Geol 100:485–522
Simon AC, Pettke T, Candela PA, Piccoli PM, Heinrich CA (2006) Copper partitioning in a melt-vapor-brine-magnetite-pyrrhotite assemblage. Geochim Cosmochim Acta 70:5583–5600
Smith IEM, Worthington TJ, Stewart RB, Price RC, Gamble J (2003) Felsic volcanism in the Kermadec arc, SW Pacific: crustal recycling in an oceanic setting. In: Larter RD, Leat PT (eds), Intra-oceanic subduction systems: tectonic and magmatic processes. Geol. Soc. London, Spec. Pub, vol 219, pp 99–118
Smyth DJ, Wood BJ, Kiseeva ES (2017) The S content of silicate melts at sulfide saturation: new experiments and a model incorporating the effects of sulfide composition. Am Miner 102:795–803
Stoffers P, Wright I, Ackermand D, Arpe T, Battershill C, Blanz T, Britten K, Both RA, Browne P, Cheminee J-L, de Ronde C, Fricke H-W, Garbe-Schönberg D, Gennerich H-H, Haase K, Hannington M, Heesemann B, Hekinian R, Herzig P, Hissmann K, Huber R, Kaul N, Lichowski F, Mitchell J, Muehlhan N, Robertson J, Rogers T, Schauer J, Schmitt M, Scholten J, Schwarz U, Smith I, Villinger H, Wilcox S, Winn K, Worthington T (1998) Report and preliminary results of RV sonne cruise SO135, Suva, Fiji-Wellington, September 9-October 15. Havre Trough—Taupo Volcanic Zone: Tectonic, magmatic and hydrothermal processes. pp 1–77
Sun W, Arculus RJ, Kamenetsky VS, Binns RA (2004) Release of gold-bearing fluids in convergent margin magmas prompted by magnetite crystallization. Nature 431:975–978
Thal J, Tivey M, Yoerger DR, Bach W (2016) Subaqueous cryptodome eruption, hydrothermal activity and related seafloor morphologies on the andesitic North Su volcano. J Volcanol Geotherm Res 323:80–96
Timm C, de Ronde CEJ, Leybourne M, Layton-Matthews D, Graham J (2012) Sources of chalcophile and siderophile elements in Kermadec arc lavas. Econ Geol 107:1527–1538
Turner S, Hawkesworth C, Rogers N, Bartlett J, Worthington T, Hergt J, Pearce J, Smith I (1997) U-238-Th-230 disequilibria, magma petrogenesis, and flux rates beneath the depleted Tonga-Kermadec island arc. Geochim Cosmochim Acta 61:4855–4884
Vallier TL, Jenner GA, Frey FA, Gill JB, Volpe AM, Hawkins JW, Morris JD, Cawood PA, Morton JL, Scholl DW, Rautenschlein M, White WM, Williams RW, Stevenson AJ, White LD (1991) Subalkaline andesite from Valu Fa Ridge, a back-arc spreading center in the southern Lau Basin; petrogenesis, comparative chemistry, and tectonic implications. Chem Geol 91:227–256
van Achterbergh E, Ryan CG, Griffin WL (2000) On-line Interactive Data Reduction for LA-ICPMS. Macquarie Research Ltd
Wallace PJ (2005) Volatiles in subduction zone magmas: concentrations and fluxes based on melt inclusion and volcanic gas data. J Volcanol Geoth Res 140:217–240
Williams-Jones AE, Heinrich CA (2005) Vapor transport of metals and the formation of magmatic-hydrothermal ore deposits. Econ Geol 100:1287–1312
Wright IC, Gamble JA (1999) Southern Kermadec submarine caldera arc volcanoes (SW Pacific): caldera formation by effusive and pyroclastic eruption. Mar Geol 161:207–227
Wright IC, Parson LM, Gamble JA (1996) Evolution and interaction of migrating cross-arc volcanism and backarc rifting: an example from the southern Havre Trough (35° 20′–37°S). J Geophys Res 101:22071–22086
Wright IC, de Ronde CEJ, Faure K, Gamble JA (1998) Discovery of hydrothermal sulfide mineralization from southern Kermadec arc volcanoes (SW Pacific). Earth Planet Sci Lett 164:335–343
Wysoczanski RJ, Wright IC, Gamble JA, Hauri EH, Luhr JF, Eggins SM, Handler MR (2006) Volatile contents of Kermadec Arc-Havre Trough pillow glasses: fingerprinting slab-derived aqueous fluids in the mantle sources of arc and back-arc lavas. J Volcanol Geoth Res 152:51–73
Wysoczanski RJ, Handler MR, Schipper CI, Leybourne MI, Creech J, Rotella MD, Nichols ARL, Wilson CJN, Stewart RB (2012) The tectonomagmatic source of ore metals and volatile elements in the southern Kermadec Arc. Econ Geol 107:1539–1556
Yang K, Scott SD (1996) Possible contribution of a metal-rich fluid to a sea-floor hydrothermal system. Nature 383:420–423
Yang K, Scott SD (2002) Magmatic degassing of volatiles and ore metals into a hydrothermal system on the modern sea floor of the eastern manus back-arc basin, Western Pacific. Econ Geol 97:1079–1100
Yang KH, Scott SD (2005) Vigorous exsolution of volatiles in the magma chamber beneath a hydrothermal system on the modern sea floor of the eastern Manus back-arc basin, western Pacific: evidence from melt inclusions. Econ Geol 100:1085–1096
Yang AY, Zhou M-F, Zhao T-P, Deng X-G, Qi L, CXu J-F (2014) Chalcophile elemental compositions of MORBs from the ultraslow-spreading Southwest Indian Ridge and controls of lithospheric structure on S-saturated differentiation. Chem Geol 382:1–13
Yeats CJ, Parr JM, Binns RA, Gemmell JB, Scott SD (2014) The SuSu Knolls hydrothermal field, eastern manus basin, papua new guinea: an active submarine high-sulfidation copper–gold system. Econ Geol 109:2207–2226
Zajacz Z, Halter W (2009) Copper transport by high temperature, sulfur-rich magmatic vapour; evidence from silicate melt and vapour inclusions in a basaltic andesite from Villarrica volcano. Earth Planet Sci Lett 282:115–121
Zajacz Z, Halter WE, Pettke T, Guillong M (2008) Determination of fluid/melt partitioning coefficients by LA-ICPMS analysis of co-existing fluid and silicate melt inclusions: controls on element partitioning. Geochim Cosmochim Acta 72:2169–2197
Zajacz Z, Seo JH, Candela PA, Piccoli PA, Tossell JA (2011) The solubility of copper in high-temperature magmatic vapors: a quest for the significance of various chloride and sulfide complexes. Geochim Cosmochim Acta 75:2811–2827
Acknowledgements
We thank C. Abe and H. Brätz for their support during work with the electron microprobe and the Laser Ablation ICP-MS, respectively. We acknowledge the constructive review of C. G. C. Patten. The study was funded by the Bundesanstalt für Geowissenschaften und Rohstoffe (Germany) and the UK Natural Environment Research Council (NERC); Minerals Security of Supply (SoS) Grant NE/ M010848/1, Tellurium and Selenium Cycling and Supply (TeaSe).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Timothy L. Grove.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Keith, M., Haase, K.M., Klemd, R. et al. Constraints on the source of Cu in a submarine magmatic-hydrothermal system, Brothers volcano, Kermadec island arc. Contrib Mineral Petrol 173, 40 (2018). https://doi.org/10.1007/s00410-018-1470-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-018-1470-5