Abstract
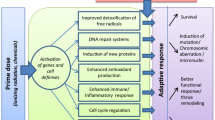
Radiation-induced adaptive response belongs to the group of non-targeted effects that do not require direct exposure of the cell nucleus by radiation. It is described as the reduced damaging effect of a challenging radiation dose when induced by a previous low priming dose. Adaptive responses have been observed in vitro and in vivo using various indicators of cellular damage, such as cell lethality, chromosomal aberrations, mutation induction, radiosensitivity, and DNA repair. Adaptive response can be divided into three successive biological phenomena, the intracellular response, the extracellular signal, and the maintenance. The intracellular response leading to adaptation of a single cell is a complex biological process including induction or suppression of gene groups. An extracellular signal, the nature of which is unknown, may be sent by the affected cell to neighbouring cells causing them to adapt as well. This occurs either by a release of diffusible signalling molecules or by gap-junction intercellular communication. Adaptive response can be maintained for periods ranging from of a few hours to several months. Constantly increased levels of reactive oxygen species (ROS) or nitric oxide (NO) have been observed in adapted cells and both factors may play a role in the maintenance process. Although adaptive response seems to function by an on/off principle, it is a phenomenon showing a high degree of inter- and intraindividual variability. It remains to be seen to what extent adaptive response is functional in humans at relevant dose and dose-rate exposures. A better understanding of adaptive response and other non-targeted effects is needed before they can be confirmed as risk estimate factors for the human population at low levels of ionising radiation.

Similar content being viewed by others
References
Olivieri G, Bodycote J, Wolff S (1984) Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 223:594–597
Iyer R, Lehnert BE (2002) Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat Res 503:1–9
Iyer R, Lehnert BE (2002) Alpha-particle-induced increases in the radioresistance of normal human bystander cells. Radiat Res 157:3–7
UNSCEAR, Non-targeted and delayed effects of exposure to ionizing radiation (2004) United Nations Scientific Committee on the Effects of Atomic Radiation Vienna
Coates PJ, Lorimore SA, Wright EG (2004) Damaging and protective cell signalling in the untargeted effects of ionizing radiation. Mutat Res 568:5–20
Cai L, Liu SZ (1990) Induction of cytogenetic adaptive response of somatic and germ cells in vivo and in vitro by low-dose X-irradiation. Int J Radiat Biol 58:187–194
Cai L (1999) Research of the adaptive response induced by low-dose radiation: where have we been and where should we go? Hum Exp Toxicol 18:419–425
Yonezawa M, Misonoh J, Hosokawa Y (1996) Two types of X-ray-induced radioresistance in mice: presence of 4 dose ranges with distinct biological effects. Mutat Res 358:237–243
Wolff S (1998) The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect 106(Suppl 1):277–283
Gajendiran N, Tanaka K, Kumaravel TS, Kamada N (2001) Neutron-induced adaptive response studied in G0 human lymphocytes using the comet assay. J Radiat Res (Tokyo) 42:91–101
Cramers P, Atanasova P, Vrolijk H, Darroudi F, Van Zeeland AA, Huiskamp R, Mullenders LH, Kleinjans JC (2005) Pre-exposure to low doses: modulation of X-ray-induced dna damage and repair? Radiat Res 164:383–390
Shadley JD, Wiencke JK (1989) Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol 56:107–118
Azzam EI, Raaphorst GP, Mitchel RE (1994) Radiation-induced adaptive response for protection against micronucleus formation and neoplastic transformation in C3H 10T1/2 mouse embryo cells. Radiat Res 138:S28–S31
Sawant SG, Randers-Pehrson G, Metting NF, Hall EJ (2001) Adaptive response and the bystander effect induced by radiation in C3H 10T(1/2) cells in culture. Radiat Res 156:177–180
Wolff S, Afzal V, Jostes RF, Wiencke JK (1993) Indications of repair of radon-induced chromosome damage in human lymphocytes: an adaptive response induced by low doses of X-rays. Environ Health Perspect 101(Suppl 3):73–77
Wolff S, Jostes R, Cross FT, Hui TE, Afzal V, Wiencke JK (1991) Adaptive response of human lymphocytes for the repair of radon-induced chromosomal damage. Mutat Res 250:299–306
Vijayalaxmi, Leal BZ, Deahl TS, Meltz ML (1995) Variability in adaptive response to low dose radiation in human blood lymphocytes: consistent results from chromosome aberrations and micronuclei. Mutat Res 348:45–50
Bosi A, Olivieri G (1989) Variability of the adaptive response to ionizing radiations in humans. Mutat Res 211:13–17
Kalina I, Nemethova G (1997) Variability of the adaptive response to low dose radiation in peripheral blood lymphocytes of twins and unrelated donors. Folia Biol (Praha) 43:91–95
Sankaranarayanan K, Von Duyn A, Loos MJ, Natarajan AT (1989) Adaptive response of human lymphocytes to low-level radiation from radioisotopes or X-rays. Mutat Res 211:7–12
Zhang L (1995) Cytogenetic adaptive response induced by pre-exposure in human lymphocytes and marrow cells of mice. Mutat Res 334:33–37
Sasaki MS (1995) On the reaction kinetics of the radioadaptive response in cultured mouse cells. Int J Radiat Biol 68:281–291
Shadley JD, Afzal V, Wolff S (1987) Characterization of the adaptive response to ionizing radiation induced by low doses of X rays to human lymphocytes. Radiat Res 111:511–517
Hain J, Jaussi R, Burkart W (1992) Lack of adaptive response to low doses of ionizing radiation in human lymphocytes from five different donors. Mutat Res 283:137–144
Wojcik A, Bonk K, Streffer C (1993) Is there an adaptive response in spleen lymphocytes of C57B1/6 mice as assessed by chromosomal aberrations? Radiat Res 135:249–256
Sorensen KJ, Attix CM, Christian AT, Wyrobek AJ, Tucker JD (2002) Adaptive response induction and variation in human lymphoblastoid cell lines. Mutat Res 519:15–24
Kelsey KT, Memisoglu A, Frenkel D, Liber HL (1991) Human lymphocytes exposed to low doses of X-rays are less susceptible to radiation-induced mutagenesis. Mutat Res 263:197–201
(UNSCEAR) UNSCotEoAR, Sources and effects of Ionizing Radiation (2000) United Nations, New York
ICRP (2004) Committee 1 Task Group Report: Low-dose Extrapolation of Radiation-Related Cancer Risk
Ina Y, Tanooka H, Yamada T, Sakai K (2005) Suppression of thymic lymphoma induction by life-long low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res 163:153–158
Mitchel RE, Jackson JS, Morrison DP, Carlisle SM (2003) Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res 159:320–327
Mitchel RE, Jackson JS, Carlisle SM (2004) Upper dose thresholds for radiation-induced adaptive response against cancer in high-dose-exposed, cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res 162:20–30
Hooker AM, Bhat M, Day TK, Lane JM, Swinburne SJ, Morley AA, Sykes PJ (2004) The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat Res 162:447–452
Ulsh BA, Miller SM, Mallory FF, Mitchel RE, Morrison DP, Boreham DR (2004) Cytogenetic dose-response and adaptive response in cells of ungulate species exposed to ionizing radiation. J Environ Radioact 74:73–81
Franca EP, Ribero CC, Teitakowski M, Londres H, Santos PL, Albuquerque HA (1965) Survey of radioactive content of food grown on Brazilian areas of high natural radioactivity. Health Phys 11:1471–1484
Chen D, Wei L (1991) Chromosome aberration, cancer mortality and hormetic phenomena among inhabitants in areas of high background radiation in China. J Radiat Res (Tokyo) 32(Suppl 2):46–53
Cheriyan VD, Kurien CJ, Das B, Ramachandran EN, Karuppasamy CV, Thampi MV, George KP, Kesavan PC, Koya PK, Chauhan PS (1999) Genetic monitoring of the human population from high-level natural radiation areas of Kerala on the southwest coast of India. II. Incidence of numerical and structural chromosomal aberrations in the lymphocytes of newborns. Radiat Res 152:S154–S158
Ghiassi-nejad M, Mortazavi SM, Cameron JR, Niroomand-rad A, Karam PA (2002) Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Phys 82:87–93
Masoomi JR, Mohammadi S, Amini M, Ghiassi-Nejad M (2006) High background radiation areas of Ramsar in Iran: evaluation of DNA damage by alkaline single cell gel electrophoresis (SCGE). J Environ Radioact 86:176–186
Ghiassi-Nejad M, Zakeri F, Assaei RG, Kariminia A (2004) Long-term immune and cytogenetic effects of high level natural radiation on Ramsar inhabitants in Iran. J Environ Radioact 74:107–116
Tao Z, Zha Y, Akiba S, Sun Q, Zou J, Li J, Liu Y, Kato H, Sugahara T, Wei L (2000) Cancer mortality in the high background radiation areas of Yangjiang, China during the period between 1979 and 1995. J Radiat Res (Tokyo) 41(Suppl):31–41
Liu SZ, Liu WH, Sun JB (1987) Radiation hormesis: its expression in the immune system. Health Phys 52:579–583
Barquinero JF, Barrios L, Caballin MR, Miro R, Ribas M, Subias A, Egozcue J (1995) Occupational exposure to radiation induces an adaptive response in human lymphocytes. Int J Radiat Biol 67:187–191
Gourabi H, Mozdarani H (1998) A cytokinesis-blocked micronucleus study of the radioadaptive response of lymphocytes of individuals occupationally exposed to chronic doses of radiation. Mutagenesis 13:475–480
Thierens H, Vral A, Barbe M, Meijlaers M, Baeyens A, Ridder LD (2002) Chromosomal radiosensitivity study of temporary nuclear workers and the support of the adaptive response induced by occupational exposure. Int J Radiat Biol 78:1117–1126
Padovani L, Appolloni M, Anzidei P, Tedeschi B, Caporossi D, Vernole P, Mauro F (1995) Do human lymphocytes exposed to the fallout of the Chernobyl accident exhibit an adaptive response? 1. Challenge with ionizing radiation. Mutat Res 332:33–38
Tedeschi B, Caporossi D, Vernole P, Padovani L, Appolloni M, Anzidei P, Mauro F (1995) Do human lymphocytes exposed to the fallout of the Chernobyl accident exhibit an adaptive response? 2. Challenge with bleomycin. Mutat Res 332:39–44
Szumiel I (2005) Adaptive response: stimulated DNA repair or decreased damage fixation? Int J Radiat Biol 81:233–241
Sasaki MS, Ejima Y, Tachibana A, Yamada T, Ishizaki K, Shimizu T, Nomura T (2002) DNA damage response pathway in radioadaptive response. Mutat Res 504:101–118
Cai L, Jiang J (1995) Mild hyperthermia can induce adaptation to cytogenetic damage caused by subsequent X irradiation. Radiat Res 143:26–33
Broome EJ, Brown DL, Mitchel RE (2002) Dose responses for adaption to low doses of (60)Co gamma rays and (3)H beta particles in normal human fibroblasts. Radiat Res 158:181–186
Morgan WF (2003) Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res 159:581–596
Morgan WF (2003) Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res 159:567–580
Emerit I, Oganesian N, Arutyunian R, Pogossian A, Sarkisian T, Cernjavski L, Levy A, Feingold J (1997) Oxidative stress-related clastogenic factors in plasma from Chernobyl liquidators: protective effects of antioxidant plant phenols, vitamins and oligoelements. Mutat Res 377:239–246
Davies MJ (2003) Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun 305:761–770
Balcer-Kubiczek EK, Harrison GH, Xu JF, Gutierrez PL (2002) Coordinate late expression of trefoil peptide genes (pS2/TFF1 and ITF/TFF3) in human breast, colon, and gastric tumor cells exposed to X-rays. Mol Cancer Ther 1:405–415
Azzam EI, De Toledo SM, Spitz DR, Little JB (2002) Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res 62:5436–5442
Mothersill C, Stamato TD, Perez ML, Cummins R, Mooney R, Seymour CB (2000) Involvement of energy metabolism in the production of ‘bystander effects’ by radiation. Br J Cancer 82:1740–1746
Burlakova EB, Mikhailov VF, azurik VK (2001) The redox homeostasis system in radiation-induced genomic instability. Radiats Biol Radioecol 41:489–499
Kadhim MA, Moore SR, Goodwin EH (2004) Interrelationships amongst radiation-induced genomic instability, bystander effects, and the adaptive response. Mutat Res 568:21–32
Mothersill C, Seymour C (2004) Radiation-induced bystander effects and adaptive responses-the Yin and Yang of low dose radiobiology? Mutat Res 568:121–128
Matsumoto H, Ohnishi T (2004) Contribution of radiation-induced, nitric oxide-mediated bystander effect to radiation-induced adaptive response. Biol Sci Space 18:108–109
Zhou H, Randers-Pehrson G, Waldren CA, Hei TK (2004) Radiation-induced bystander effect and adaptive response in mammalian cells. Adv Space Res 34:1368–1372
Ko M, Lao XY, Kapadia R, Elmore E, Redpath JL (2006) Neoplastic transformation in vitro by low doses of ionizing radiation: role of adaptive response and bystander effects. Mutat Res 597:11–17
Redpath JL, Liang D, Taylor TH, Christie C, Elmore E (2001) The shape of the dose-response curve for radiation-induced neoplastic transformation in vitro: evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res 156:700–707
Joiner MC, Marples B, Lambin P, Short SC, Turesson I (2001) Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys 49:379–389
Joiner MC, Lambin P, Malaise EP, Robson T, Arrand JE, Skov KA, Marples B (1996) Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res 358:171–183
Marples B, Joiner MC (1995) The elimination of low-dose hypersensitivity in Chinese hamster V79-379A cells by pretreatment with X rays or hydrogen peroxide. Radiat Res 141:160–169
Short SC, Kelly J, Mayes CR, Woodcock M, Joiner MC (2001) Low-dose hypersensitivity after fractionated low-dose irradiation in vitro. Int J Radiat Biol 77:655–664
Wouters BG, Skarsgard LD (1997) Low-dose radiation sensitivity and induced radioresistance to cell killing in HT-29 cells is distinct from the “adaptive response” and cannot be explained by a subpopulation of sensitive cells. Radiat Res 148:435–442
Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC (2004) Low-dose hyper-radiosensitivity: a consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat Res 161:247–255
Wykes SM, Piasentin E, Joiner MC, Wilson GD, Marples B (2006) Low-dose hyper-radiosensitivity is not caused by a failure to recognize DNA double-strand breaks. Radiat Res 165:516–524
Mothersill C, Seymour CB, Joiner MC (2002) Relationship between radiation-induced low-dose hypersensitivity and the bystander effect. Radiat Res 157:526–532
Wiencke JK, Afzal V, Olivieri G, Wolff S (1986) Evidence that the [3H] thymidine-induced adaptive response of human lymphocytes to subsequent doses of X-rays involves the induction of a chromosomal repair mechanism. Mutagenesis 1:375–380
Youngblom JH, Wiencke JK, Wolff S (1989) Inhibition of the adaptive response of human lymphocytes to very low doses of ionizing radiation by the protein synthesis inhibitor cycloheximide. Mutat Res 227:257–261
Kim JH, Hahm KH, Cho CK, Yoo SY (1996) Protein biosynthesis in low dose ionizing radiation-adapted human melanoma cells. J Radiat Res (Tokyo) 37:161–169
Bravard A, Luccioni C, Moustacchi E, Rigaud O (1999) Contribution of antioxidant enzymes to the adaptive response to ionizing radiation of human lymphoblasts. Int J Radiat Biol 75:639–645
Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR (2004) STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci USA 101:1714–1719
Klokov D, Criswell T, Leskov KS, Araki S, Mayo L, Boothman DA (2004) IR-inducible clusterin gene expression: a protein with potential roles in ionizing radiation-induced adaptive responses, genomic instability, and bystander effects. Mutat Res 568:97–110
Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA (2003) Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem 278:11590–11600
Woloschak GE, Chang-Liu CM, Jones PS, Jones CA (1990) Modulation of gene expression in Syrian hamster embryo cells following ionizing radiation. Cancer Res 50:339–344
Ikushima T, Aritomi H, Morisita J (1996) Radioadaptive response: efficient repair of radiation-induced DNA damage in adapted cells. Mutat Res 358:193–198
Hendrikse AS, Hunter AJ, Keraan M, Blekkenhorst GH (2000) Effects of low dose irradiation on TK6 and U937 cells: induction of p53 and its role in cell-cycle delay and the adaptive response. Int J Radiat Biol 76:11–21
Morimoto S, Gordon A, Fukunishi N, Yatagai F (2001) Cellular responses by exposure to heavy-ions. Adv Space Res 27:401–409
Coleman MA, Yin E, Peterson LE, Nelson D, Sorensen K, Tucker JD, Wyrobek AJ (2005) Low-dose irradiation alters the transcript profiles of human lymphoblastoid cells including genes associated with cytogenetic radioadaptive response. Radiat Res 164:369–382
Aghamohammadi SZ, Savage JR (1992) The effect of X-irradiation on cell cycle progression and chromatid aberrations in stimulated human lymphocytes using cohort analysis studies. Mutat Res 268:223–230
Wojcik A, Aghamohammadi S, Aillaud M, Bosi A, Dai G, Olivieri G, Salone B, Savage JR, Shadley JD, Streffer C (1996) Adaptive response to ionizing radiation in human lymphocytes: the problem of scoring aberrations in cells irradiated during asynchronous growth. Mutat Res 366:137–143
Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A (1988) Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med 53:39–47
Aghamohammadi SZ, Savage JR (1991) A BrdU pulse double-labelling method for studying adaptive response. Mutat Res 251:133–141
Wang ZQ, Saigusa S, Sasaki MS (1991) Adaptive response to chromosome damage in cultured human lymphocytes primed with low doses of X-rays. Mutat Res 246:179–186
Amundson SA, Bittner M, Meltzer P, Trent J, Fornace AJ Jr (2001) Biological indicators for the identification of ionizing radiation exposure in humans. Expert Rev Mol Diagn 1:211–219
Salone B, Pretazzoli V, Bosi A, Olivieri G (1996) Interaction of low-dose irradiation with subsequent mutagenic treatment: role of mitotic delay. Mutat Res 358:155–160
Barcellos-Hoff MH, Costes SV (2006) A systems biology approach to multicellular and multi-generational radiation responses. Mutat Res 597:32–38
Pogribny I, Koturbash I, Tryndyak V, Hudson D, Stevenson SM, Sedelnikova O, Bonner W, Kovalchuk O (2005) Fractionated low-dose radiation exposure leads to accumulation of DNA damage and profound alterations in DNA and histone methylation in the murine thymus. Mol Cancer Res 3:553–561
Pogribny I, Raiche J, Slovack M, Kovalchuk O (2004) Dose-dependence, sex-and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem Biophys Res Commun 320:1253–1261
Kaup S, Grandjean V, Mukherjee R, Kapoor A, Keyes E, Seymour CB, Mothersill CE, Schofield PN (2006) Radiation-induced genomic instability is associated with DNA methylation changes in cultured human keratinocytes. Mutat Res 597:87–97
Gonzalgo ML, Jones PA (1997) Mutagenic and epigenetic effects of DNA methylation. Mutat Res 386:107–118
Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R (2003) Induction of tumors in mice by genomic hypomethylation. Science 300:489–492
Esteller M (2005) Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol 45:629–656
Bandyopadhyay D, Medrano EE (2003) The emerging role of epigenetics in cellular and organismal aging. Exp Gerontol 38:1299–1307
Kovalchuk I, Abramov V, Pogribny I, Kovalchuk O (2004) Molecular aspects of plant adaptation to life in the Chernobyl zone. Plant Physiol 135:357–363
Kovalchuk O, Burke P, Arkhipov A, Kuchma N, James SJ, Kovalchuk I, Pogribny I (2003) Genome hypermethylation in Pinus silvestris of Chernobyl-a mechanism for radiation adaptation? Mutat Res 529:13–20
Glasspool RM, Teodoridis JM, Brown R (2006) Epigenetics as a mechanism driving polygenic clinical drug resistance. Br J Cancer 94:1087–1092
Acknowledgments
Dr. Shin Saigusa is acknowledged for his supportive suggestions and Dr. Linda Walsh and Dr. Thomas Jung for critically reading the manuscript. The work has been supported by the German Federal Ministry of Environment, Nature Preservation and Reactor Safety and the German Federal Office of Radiation Protection under contract number StSch 4367.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tapio, S., Jacob, V. Radioadaptive response revisited. Radiat Environ Biophys 46, 1–12 (2007). https://doi.org/10.1007/s00411-006-0078-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-006-0078-8