Abstract
Cerebrospinal fluid (CSF) neurofilament light chain (NfL) has emerged as putative diagnostic biomarker in amyotrophic lateral sclerosis (ALS), but it remains a matter of debate, whether CSF total tau (ttau), tau phosphorylated at threonine 181 (ptau) and the ptau/ttau ratio could serve as diagnostic biomarker in ALS as well. Moreover, the relationship between CSF NfL and tau measures to further axonal and (neuro)degeneration markers still needs to be elucidated. Our analysis included 89 ALS patients [median (range) age 63 (33–83) years, 61% male, disease duration 10 (0.2–190) months] and 33 age- and sex-matched disease controls [60 (32–76), 49%]. NfL was higher and the ptau/ttau ratio was lower in ALS compared to controls [8343 (1795–35,945) pg/ml vs. 1193 (612–2616), H(1) = 70.8, p < 0.001; mean (SD) 0.17 (0.04) vs. 0.2 (0.03), F(1) = 14.3, p < 0.001], as well as in upper motor neuron dominant (UMND, n = 10) compared to classic (n = 46) or lower motor neuron dominant ALS [n = 31; for NfL: 16,076 (7447–35,945) vs. 8205 (2651–35,138) vs. 8057 (1795–34,951)], Z ≥ 2.5, p ≤ 0.01; for the ptau/ttau ratio: [0.13 (0.04) vs. 0.17 (0.04) vs. 0.18 (0.03), p ≤ 0.02]. In ALS, NfL and the ptau/ttau ratio were related to corticospinal tract (CST) fractional anisotropy (FA) and radial diffusivity (ROI-based approach and whole-brain voxelwise analysis). Factor analysis of mixed data revealed a co-variance pattern between NfL (factor load − 0.6), the ptau/ttau ratio (0.7), CST FA (0.8) and UMND ALS phenotype (− 2.8). NfL did not relate to any further neuroaxonal injury marker (brain volumes, precentral gyrus thickness, peripheral motor amplitudes, sonographic cross-sectional nerve area), but a lower ptau/ttau ratio was associated with whole-brain gray matter atrophy and widespread white matter integrity loss. Higher NfL baseline levels were associated with greater UMN disease burden, more rapid disease progression, a twofold to threefold greater hazard of death and shorter survival times. The findings that higher CSF NfL levels and a reduced ptau/ttau ratio are more associated with clinical UMN involvement and with reduced CST FA offer strong converging evidence that both are markers of central motor degeneration. Furthermore, NfL is a marker of poor prognosis, while a low ptau/ttau ratio indicates extramotor pathology in ALS.
Similar content being viewed by others
Introduction
Cerebrospinal fluid (CSF) neurofilament light chain (NfL) has emerged as putative diagnostic biomarker in several neurodegenerative conditions [1, 2], such as amyotrophic lateral sclerosis (ALS), and ALS patients reveal significantly higher levels compared to controls or disease mimics [3,4,5,6,7,8,9]. CSF neurofilaments seem also to aid as a prognostic biomarker [4, 5, 8,9,10,11] and have been found to be higher in ALS patients with dominant upper motor neuron (UMN) involvement [4, 5, 12]. In the meanwhile, several studies have also taken account of serum NfL which has additionally proven great potential to discriminate between ALS and controls or disease mimics [7, 13, 14]. Compared to CSF, serum neurofilament might, however, be less sensitive towards clinical and electrophysiological measures of motor neuron degeneration [9, 15], emphasizing the pivotal role of CSF neurofilaments when taking account of the extent of neuroaxonal damage despite less convenient sampling. With the exception of few studies focusing on the corticospinal tract’s (CST) integrity applying diffusion tensor imaging (DTI) there is, however, nearly no data available how CSF neurofilaments relate to other biomarkers of peripheral (PNS) and central nervous system (CNS) neuroaxonal injury in ALS [6, 16]. Understanding their certain biomarker associations would indeed even improve the leading candidate role of CSF neurofilaments to aid as a promising outcome measure in future ALS therapeutic trials [17].
In contrast to CSF NfL, it is a matter of debate whether CSF total tau (ttau) and tau phosphorylated at threonine 181 (ptau) could serve as diagnostic biomarker in ALS as well, and there are just as many studies reporting elevated (abnormal) or normal levels in ALS when compared to controls [12, 18,19,20,21,22,23,24,25]. Two studies further proposed a reduction of the ptau/ttau ratio in ALS [23, 24]. While CSF tau seems not to universally correlate with disease progression in ALS [12, 19, 21, 26], few studies indeed found a relationship between higher CSF ttau or ptau at baseline and worse motor function [6, 23]. Moreover, as for CSF NfL, with the exception of two DTI studies reporting an association between CST integrity and the ptau/ttau ratio, but not with ttau or ptau, there are no data available how tau measures relate to further neuroaxonal injury markers in ALS [6, 23]. Continuing studies are thus indeed needed to determine the biomarker role of ttau and ptau in ALS.
We thus here conducted several analyses taking especially into account how CSF NfL, ttau, ptau and the ptau/ttau ratio distribute within certain ALS subgroups, relate to further axonal and (neuro)degeneration markers such as motor amplitudes or precentral gyrus thickness and the ALS patients’ long-term outcome using a retrospective approach.
Methods
ALS sample
Our study comprised 89 ALS patients recruited from the Departments of Neurology, Otto-von-Guericke University, Magdeburg and Hannover Medical School, Hannover, Germany. Patients were diagnosed by one of two experienced neurologists (S. V., S. P.) according to the revised El Escorial criteria comprising the assessment of the number of regions (bulbar, thoracic, upper limb, lower limb) with UMN (clinically) or lower motor neuron (LMN) involvement (clinically or via electromyography) [27]. Similar to previous studies, we also included patients presenting with LMN signs only (“suspected ALS”) [14]. The Penn UMN score was recorded to assess the UMN disease burden in the bulbar segment as well as in each of the four limbs [28] (see Supplemental). ALS clinical phenotypes were classified in line with operational definitions as specified previously [29, 30] (see Supplemental). At baseline, patients underwent a clinical and diagnostic workup (ALS functional rating scale (ALSFRS-R) total score, genetic testing, CSF measures of NfL, ttau, ptau, total protein and the CSF albumin/serum albumin ratio (Qalb × 10−3); for methodological details regarding CSF measurements and the performance of the NfL assay see Supplemental and Supplemental Table 1). Measures of neuroaxonal injury comprised those PNS and CNS markers commonly found to be altered in ALS: median and ulnar nerve compound motor action potential (CMAP) amplitudes [31, 32] and sonographic cross-sectional nerve area (CSA) [29, 33] (for methodological details of PNS measures see [29]), precentral gyrus thickness, cortical and subcortical cerebral gray matter (GM) volumes and CST DTI metrics [e.g., fractional anisotropy (FA)] [34,35,36,37]. Disease duration was the time in months between symptom onset and a patient’s baseline visit. Disease progression rate (DPR) was determined as (48-ALSFRS-R)/disease duration (points per month). Patients underwent follow-up ALSFRS-R measurements within a mean (SD) time interval of 6 (8) months. Please see the Supplemental and Supplemental Fig. 1 for the detailed demonstration of the availability of all measures.
Controls
Cross-sectional CSF NfL, ttau and ptau measures were additionally conducted in a hospital-based cohort of 33 neurologic patients (non-motor neuron disease controls), comprising cases with non-specific complaints who underwent lumbar puncture in terms of a diagnostic workup to rule out any neurologic condition. None of those disease controls suffered from any neuromuscular disorders (i.e., peripheral polyneuropathies, muscle or motor neuron disease) nor did they display any specific abnormalities on the neurological exam. CSF NfL data were available in all subjects, while tau measures have been conducted in 16 out of the 33 control cases only (please see Supplemental Table 2 for further details).
Standard protocol approvals, registrations, and patient consents
The study was approved by the local ethics committee (No. 150/09, No. 07/17, No. 16/17), and all subjects gave written informed consent.
3T MRI measures of the brain
All MRI sessions were performed on the same Siemens Verio 3 T system (Siemens Medical Systems, Erlangen, Germany) at the same site (Magdeburg), and all patients underwent exactly the same MRI protocol. 3D MPRAGE images were acquired [for bilateral precentral gyrus thickness, GM (GMV) and white matter (WMV) and total brain (TBV) volumes]. Diffusion MRI data were used to compute the maps of DTI scalars [FA, mean diffusivity (MD), radial diffusivity (RD), axial diffusivity (AD)]. Applying tract-based spatial statistics [38] whole-brain regression analysis with white matter hyperintensities (WMH) as covariate of no interest (assessed in a T2-weighted FLASH sequence according to the Fazekas scale [39]) was conducted (with the Randomise tool version 2.9 available in FSL, 5000 permutations, threshold-free cluster enhancement (TFCE), 2D optimization for tract-based DTI analysis). Individual median values of bilateral CST DTI scalars were additionally extracted (region of interest (ROI) analysis). For the in-depth demonstration of all imaging analyses see the Supplemental.
Statistics
Gaussian distribution of data was assessed using the Shapiro–Wilk test. For group comparisons, for non-normally distributed data Kruskal–Wallis one-way analysis of variance (ANOVA) with post hoc pairwise Mann–Whitney U testing, and for normally distributed data ANOVA with Bonferroni post hoc testing was conducted. Relationship between distinct variables was calculated using bivariate correlations. Left- and right-sided CMAP amplitude and CSA measures were averaged, as there were no side differences. Compared to the left side, the right motor cortex was significantly thinner (Z = − 5.0, p < 0.001, Wilcoxon signed-rank test), which is a common finding in ALS [40]; left- and right-sided measures were thus considered separately.
We then applied a factor analysis for mixed (quantitative and qualitative) data (FAMD) using FactoMineR version 1.27 [41] to capture co-variance patterns between distinct measures related to CSF NfL. We included CSF NfL, the ptau/ttau ratio, CST FA (which is the most sensitive DTI metrics in ALS [42]) and ALS phenotype into that model and extracted 1 component with an eigenvalue > 1, which explained 40% of the variance in the data.
Random intercept mixed effects linear models with CSF NfL (ttau, ptau, ptau/ttau ratio) median-split (main effect) and time (disease duration) in months (main effect) were calculated to assess CSF NfL (ttau, ptau, ptau/ttau ratio) × time interaction effects on longitudinal ALSFRS-R total score, and estimates (e) are given. In addition, Kruskal–Wallis one-way ANOVA or ANOVA was calculated to assess group effects of slow (averaged ALSFRS-R points lost per month < 0.4 from disease onset to last available ALSFRS-R), intermediate (≥ 0.4, ≤ 1.4) and fast (> 1.4) disease progressors [43] on baseline CSF NfL (ttau, ptau, ptau/ttau ratio).
Cox proportional hazard models giving the hazard ratio [Exp(B)] and Kaplan–Meier analysis using a pairwise log rank test were conducted to compare survival rates and times between ALS patients revealing low, medium or high CSF NfL (ttau, ptau, ptau/ttau ratio) levels (terciles) at baseline, and censoring was done at the date of the last follow-up.
p values < 0.05 were deemed to be statistically significant. Analyses were performed using the IBM SPSS Statistics 23.0 software.
Results
Sample
Table 1 demonstrates the demographics and the clinical data of the whole sample. Supplemental Table 3 depicts the demographics and clinical data separately for the ALS phenotypes.
CSF NfL, ttau, ptau and ptau/ttau ratio in controls and ALS
CSF NfL lacked symmetry [controls: D(33) = 0.9, p = 0.02; ALS: D(89) = 0.9, p < 0.001] but revealing a significant positive skew distribution instead with most measures clustering at the lower end of the scale (controls: zskewness = 2.6, p < 0.01; ALS: zskewness = 5.6, p < 0.001; Fig. 1a). In ALS, distribution was the same for CSF ttau and ptau [ttau: D(88) = 0.9, p < 0.001, zskewness = 4.4, p < 0.001; ptau: D(88) = 0.9, p < 0.001, zskewness = 4.1, p < 0.001], while the ptau/ttau ratio was normally distributed [D(88) = 1.0, p = 0.4, zskewness = 1.5, p > 0.05]. In controls, all CSF tau measures were normally distributed [ttau: D(14) = 0.9, p = 0.5, zskewness = − 0.7, p > 0.05; ptau: D(14) = 1.0, p = 1.0, zskewness = − 0.04, p > 0.05; ptau/ttau ratio: D(14) = 0.9, p = 0.3, zskewness = 1.3, p > 0.05] (Fig. 1b).
Quantile function of CSF NfL values and the ptau/ttau ratio within the samples under investigation. Graph demonstrates CSF NfL concentrations (a) and the ptau/ttau ratio (b) in controls (CON) and ALS. ALS patients compared to controls revealed significantly higher CSF NfL concentrations and a significantly lower ptau/ttau ratio. c demonstrates the significant relationship between CSF NfL and the ptau/ttau ratio in ALS. **p≤0.001
In ALS compared to controls, NfL was higher [median (range) 8343 (1795–35,945) pg/ml vs. 1193 (612–2616), H(1) = 70.8, p < 0.001] and the ptau/ttau ratio was lower [mean (SD) 0.17 (0.04) vs. 0.2 (0.03), F(1) = 14.3, p < 0.001; Fig. 1a, b]. There were no group differences for ttau and ptau [ALS vs. controls, ttau: 236 (96–666) pg/ml vs. 260 (122–373), ptau: 40 (17–99) pg/ml vs. 53 (20–80)].
In ALS, NfL was related to Qalb (rho = 0.2, p = 0.04), and ttau and ptau were related to age (rho = 0.4, p < 0.001, respectively). There was no association with sex, disease duration, or onset site; NfL did relate to the ptau/ttau ratio (rho = − 0.4, p < 0.001; Fig. 1c), but not to ttau and ptau.
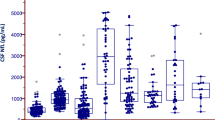
There was a significant effect of clinical phenotype on CSF NfL [H(2) = 7.9, p = 0.02] and on the CSF ptau/ttau ratio [F(2) = 6.6, p = 0.002]. Pairwise comparisons revealed group differences between classic and upper motor neuron dominant (UMND) (NfL: Z = 2.5, p = 0.01; ptau/ttau ratio: p = 0.02) or lower motor neuron dominant (LMND) and UMND ALS (NfL: Z = 2.6, p = 0.008; ptau/ttau ratio: p = 0.001), with UMND compared to classic or LMND patients displaying higher NfL [16,076 (7447–35,945) vs. 8205 (2651–35,138) vs. 8057 (1795–34,951); Fig. 2a] and a lower ptau/ttau ratio [0.13 (0.04) vs. 0.17 (0.04) vs. 0.18 (0.03); Fig. 2c]. In PLS, NfL was lower than in ALS [7043 (6454–7632)], and the ptau/ttau ratio was rather similar to the ratio in classic/LMND, but higher than in UMND ALS [0.16 (0.02)]; as there were only two PLS cases (Table 1), they were, however, not considered for group and pairwise subgroup comparisons.
CSF NfL concentrations and the ptau/ttau ratio across the ALS disease spectrum. When compared to classic and lower motor neuron dominant (LMND) ALS, upper motor neuron dominant (UMND) ALS phenotype was related to significantly higher CSF NfL concentrations (a) and a significantly lower ptau/ttau ratio (c). Higher CSF NfL levels and a smaller ptau/ttau ratio were, moreover, associated with a greater UMN disease burden as assessed by the Penn UMN score (b, d). *p ≤ 0.05, **p ≤ 0.001
There was, moreover, a significant relationship between higher NfL or a lower ptau/ttau ratio and greater UMN disease burden according to the Penn UMN score (rho = 0.4, p < 0.001, rho = − 0.2, p = 0.03; Fig. 2b, d).
Ttau and ptau did not differ across ALS phenotypes, and did not relate to the Penn UMN score.
CSF NfL, ttau, ptau, the ptau/ttau ratio and biomarkers of neuroaxonal injury in ALS
There was a medium-effect size relationship between NfL and median CST FA, MD and RD (ROI-based approach, rho = − 0.5, p = 0.001, rho = 0.3, p = 0.02, rho = 0.5, p < 0.001; Fig. 3a, c). Correlations between NfL and median CST FA and RD remained significant when solely considering the classic ALS patients (rho = − 0.4, p = 0.03, rho = 0.4, p = 0.05; please see the Supplemental and Supplemental Fig. 2). For the whole ALS cohort, strong correlation between NfL and FA along the cortical spinal pathway additionally emerged from an unbiased whole-brain analysis and it was statistically significant at the stringent threshold of p < 0.05 FWE corrected (Fig. 3b). At the same statistical threshold, the regression analysis revealed also a positive correlation between NfL and RD which spatially overlapped with the distribution of the significant results in the FA analysis (Fig. 3d).
Relationship between CSF NfL and DTI metrics in ALS. The relationship depicted between CSF NfL and median fractional anisotropy (FA) or median radial diffusivity (RD) of the corticospinal tract (CST) using a ROI-based approach is demonstrated in a, c. The results of the skeletonized whole-brain regression analysis for FA (b in red) and RD (d in blue) are overlapped to the mean FA map. The statistical threshold is set at p < 0.05 FWE corrected. The images are displayed following the radiological convention
Likewise, there was a medium-effect size relationship between the ptau/ttau ratio and median CST FA and RD (ROI-based approach, rho = 0.4, p = 0.01, rho = − 0.3, p = 0.03; Fig. 4a, c). Considering the whole-brain analysis, the correlation between the ptau/ttau ratio and DTI metrics survived the stringent FWE correction for multiple comparison (p < 0.05) and partially overlapped with the results of the correlation analysis between NfL level and DTI metrics (Figs. 3b, d, 4b, d). In both cases, the CST was involved (please see also the results of the ROI analysis, Figs. 3a, c, 4a, c), but the whole-brain analysis revealed that the ptau/ttau ratio was also related to a FA decrease in the genu of the corpus callosum, in the anterior portion of the corona radiata (bilateral), in the anterior portion of the cingulum WM (right), in the external capsule (left) and in anterior limb of the internal capsule (left) (Fig. 4b). The ptau/ttau ratio was also correlated with increased RD values in all sections of the corpus callosum (Fig. 4d).
Relationship between the CSF ptau/ttau ratio and DTI metrics in ALS. The relationship depicted between the CSF ptau/ttau ratio and median FA or RD of the CST using a ROI-based approach is demonstrated in a, c. The results of the skeletonized whole-brain regression analysis for FA (b in red) and RD (d in blue) are overlapped to the mean FA map. The statistical threshold is set at p < 0.05 FWE corrected. The images are displayed following the radiological convention
There was no relationship between NfL, ttau, ptau and the ptau/ttau ratio and WMH.
FAMD revealed a co-variance pattern between CSF NfL (factor load − 0.6), the ptau/ttau ratio (0.7), CST FA (0.8) and UMND ALS phenotype (− 2.7), which has to be interpreted this way, that high NfL together with a lower ptau/ttau ratio and CST FA decrease is found in patients with dominant UMN involvement.
A lower ptau/ttau ratio was, moreover, related to smaller GMV (r = 0.3, p = 0.02). There was no association between CSF NfL, ptau, ttau and the ptau/ttau ratio and any further PNS and CNS axonal or (neuro)degeneration ALS marker (e.g., nerve CSA, CMAP amplitudes, cortical thickness of the precentral gyrus).
CSF NfL, ttau, ptau and the ptau/ttau ratio and long-term prognosis in ALS
There was a small-effect size inverse relationship between NfL and baseline ALSFRS-R total score (rho = − 0.2, p = 0.03): ALS patients with higher compared to lower NfL (median-split) revealed lower ALSFRS-R total scores [H(1) = 4.6, p = 0.03].
Mixed effects linear models displayed a significant NfL main effect on longitudinal ALSFRS-R total score (e = − 4.9, p = 0.01), while there was no significant NfL × time interaction effect. This means that when averaging the ALSFRS-R total score across all available time points, ALS patients with higher compared to lower baseline NfL (median-split) show a − 4.9 points lower mean value.
There was a trend-level group effect of slow, intermediate and fast progressors on baseline CSF NfL [H(2) = 5.0, p = 0.08]. Post hoc analysis revealed that fast compared to intermediate progressors displayed significantly higher NfL (Z = 2.3, p = 0.02) (Fig. 5a). In line with this, there was a small-effect size correlation between NfL and DPR (rho = 0.2, p = 0.07, trend-level).
Disease progression and survival as a function of baseline CSF NfL values in ALS. Fast disease progression (averaged ALSFRS-R points lost per month > 1.4) was related to higher baseline CSF NfL levels (a). b Depicts predicted survival curves after covariate adjustment (age, sex, onset site, sporadic vs. familial ALS, baseline ALSFRS-R total score) for CSF NfL terciles (Cox proportional hazard modeling). ALS patients with high (third tercile) compared to medium (second tercile) and low (first tercile) baseline NfL levels display a twofold to threefold greater hazard of death. c demonstrates Kaplan–Meier analysis; median survival time was significantly shorter in ALS patients exhibiting baseline CSF NfL levels within the upper (third) tercile compared to patients revealing baseline CSF NfL concentrations within the medium (second) or lower tercile (first). *p < 0.05
Cox proportional hazard modeling depicted a twofold to threefold greater hazard of death for patients with high CSF NfL compared to patients having medium or low NfL [Exp(B) (95% CI) = 0.5 (0.3, 0.9), p = 0.01, Exp(B) (95% CI) = 0.3 (0.1, 0.9), p = 0.02]. Hazard remained after model adjustment for age, sex, onset site, sporadic vs. familial ALS and baseline ALSFRS-R total score [Exp(B) (95% CI) = 0.4 (0.2, 0.8), p = 0.007, Exp(B) (95% CI) = 0.3 (0.1, 0.9), p = 0.03; Fig. 5b].
Kaplan–Meier analysis was in line with those results, displaying significantly shorter median (SE) survival times of patients showing high [upper tercile, 34 (3) months] compared to medium [medium tercile, 48 (3), χ2 = 6.1, p = 0.01] or low baseline NfL [lower tercile, 45 (22), χ2 = 5.1, p = 0.02; Fig. 5c].
There was no relationship between ttau, ptau or the ptau/ttau ratio and the patients’ functional scoring (ALSFRS-R), disease progression and survival.
Discussion
Our analysis argues that CSF NfL and the ptau/ttau ratio act as diagnostic biomarkers which at once relate to one another, to UMN involvement and DTI white matter signature of cerebral CST degeneration. A smaller ptau/ttau ratio was further indicative of whole-brain gray matter atrophy and widespread microstructural white matter pathology. Neither NfL nor CSF tau measures were related to peripheral motor axon involvement. Our results, moreover, demonstrate a particular relationship between higher baseline CSF NfL and greater disease severity, more rapid disease progression, greater hazard of death and shorter survival in ALS. These findings suggest that elevated CSF NfL and a lower ptau/ttau ratio are particularly biomarkers of central motor degeneration that together with measures emerging from microstructural white matter neuroimaging could be used to stratify ALS patients and to monitor their disease progression presumably allowing to assess the efficacy of future neuroprotective therapies.
Irrespective of ALS pathophysiology, neurofilaments are structural constituents of the neuroaxonal cytoskeleton and integral components of synapses; they are essential for axonal growth, transport and signaling pathways. Neurofilaments are highly abundant in the large Betz cells of the motor cortex and in large-caliber myelinated axons, e.g., of the CST [44,45,46,47]. White matter and cortical injury is related to elevated CSF NfL that represents a downstream effect of neuroaxonal loss [48,49,50,51]. Tau is a microtubule-associated protein that is highly expressed in neuronal axons, e.g., in thin unmyelinated axons of the neocortical gray matter, providing axonal transport and maintenance of the neurons’ structure/morphology [52, 53]. Neuroaxonal degeneration results in increased release of tau from the brain into the interstitial fluid/CSF; and—like CSF NfL—elevation of CSF ttau likely reflects unspecific neuronal and axonal damage, as observed in many chronic neurodegenerative diseases [54]. High CSF ptau, however, specifically relates to the occurrence of neurofibrillary tangle formations and is one hallmark diagnostic biomarker of Alzheimer’s disease (AD) [55].
This work replicates the results of several studies showing significantly higher CSF NfL levels in ALS compared to controls (e.g., [4, 6, 14]). Our findings, moreover, support recent data that ALS relates to a lower ptau/ttau ratio, indicating a shifted proportion of ttau and ptau which might be based on three constellations: ALS patients compared to controls reveal (1) higher CSF ttau, or (2) lower CSF ptau, or both—(3) higher CSF ttau together with lower CSF ptau. Recent studies reporting a reduced ptau/ttau ratio in ALS or ALS with frontotemporal dementia (ALS-FTD), either disclosed higher CSF ttau [24, 56, 57] or lower ptau [23]. Because phosphorylation of tau occurs mainly in AD and not so much in other neurodegenerative disorders, one may suspect that a lower ptau/ttau ratio probably reflects severe neuroaxonal damage in rapidly progressive diseases favoring the hypothesis of increased CSF ttau rather than a ptau reduction [57]. In our ALS sample, however, both ttau and ptau were unaltered, indicating that the ptau/ttau ratio seems to be a more sensitive candidate biomarker in ALS than CSF ttau or ptau alone.
As shown before, in ALS CSF NfL is skewed towards lower levels, leaving the pivotal question what drives the substantial CSF NfL increase found in a certain ALS subgroup. Our data reveal that in ALS the variability of NfL depends on cerebral CST degeneration and UMN involvement, extending the findings of previous studies [4, 5, 16] by showing for the first time that those variables co-vary, implying that they are altered together in the same patient. This is supported by the fact that in ALS the strong DTI white matter signature in terms of CST FA decrease also relates to UMN pathology [28, 34, 58,59,60]. Constellation of high CSF NfL, CST degeneration and dominant UMN involvement additionally goes along with a lower ptau/ttau ratio. Supposing that a smaller ptau/ttau ratio indicates neuroaxonal injury, our findings emphasize that CSF NfL and the ptau/ttau ratio together stand for the extent and the severity of upper motor neuron degeneration in ALS. However, in contrast to NfL, a reduced ptau/ttau ratio further indicates gray matter atrophy and white matter integrity loss beyond upper motor neuron pathology. This suggests that the ratio could also serve as a marker for extramotor involvement in ALS, which needs to be elucidated within future studies.
In our sample, CSF NfL and the ptau/ttau ratio were not only related to FA, but also to various DTI metrics (MD, RD). FA and RD alterations seem to be sensitive against demyelination, Wallerian-type myelin degeneration and axonal integrity loss, especially in chronic diseases with extended axonal damage; MD changes have, moreover, been speculated to relate to augmented cellularity (e.g., due to the loss of large myelinated axons) [61, 62]. As the biological underpinnings of differences in DTI variables are still unclear, especially in terms of co-existing underlying pathologies (i.e., axonal injury, demyelination, inflammation) [62, 63], we prefer to refrain from further interpretations of those particular findings.
Our results are in line with two recent studies relating higher CSF NfL and a lower ptau/ttau ratio to altered DTI metrics in ALS [16, 23]. They, however, contradict another ALS study that did not find any relationship between CSF NfL and DTI CST integrity [6]. Steinacker et al. therein combine participants scanned on two different MRI systems and with two different field strengths (2/3 underwent a 1.5T MRI, 1/3 underwent a 3 T MRI). The authors have shown that the data obtained from the two systems were comparable and they thus combined all the DTI values in a single analysis. A lower field strength (1.5 T compared to 3 T) inherently entails a lower signal-to-noise ratio that could, potentially, mask the presence of an effect like the relationship between FA values and NfL levels. Our study, as well as the aforementioned study of Menke and colleagues [16], which also reported a correlation between both FA and RD values and levels of NfL in ALS patients, is based on data acquired on a single 3T scanner employing only one protocol. This kind of design is likely to enhance the sensitivity of the study to detect effects that could, otherwise, be masked by noise.
One might have expected to find an association between motor cortex thickness denoting UMN pathology and CSF NfL and the ptau/ttau ratio. As Betz cells and their gray matter axons just represent a small fraction of the motor cortex their degeneration seems to be better mirrored by NfL levels or the ptau/ttau ratio instead of affecting the overall number/density of motor neurons/gray matter neuropil or precentral gyrus thickness, accounting for the absent relationship [42, 64].
We failed to find a relationship between CSF NfL or the ptau/ttau ratio, clinical LMN involvement and LMN biomarkers of axonal injury (e.g., reduced CMAP amplitudes or nerve CSA indicating muscle and nerve atrophy, respectively). However, patients with LMND clinical phenotype presenting isolated LMN signs, likewisely displayed elevated NfL concentrations or a reduced ptau/ttau ratio within the range of classic ALS. ALS disease mimics with sole LMN involvement, e.g., Kennedy’s disease or spinal muscular atrophy, do contrary not show abnormal neurofilament levels [5, 14]. Our findings of significant NfL increase across all clinical phenotypes are in line with previous observations in early symptom onset ALS [14], strengthening the role of NfL and ptau/ttau as biomarkers which enhance the diagnostic accuracy of ALS, especially in patients with predominant or isolated LMN signs.
Corroborating previous findings, these analyses also indicate that in ALS higher CSF NfL refers to greater disease severity at baseline and longitudinal follow-up. It conversely remains vague whether higher baseline NfL also relates to a steeper decline or a steady trajectory of overall motor function (group effect of slow, intermediate and fast progressors on NfL vs. non-significant time × median-split baseline NfL interaction effect on longitudinal ALSFRS-R total score). Considering the latter, it might be possible that a certain ALS subject just comes into the disease with an already determined signature of functional performance and related CSF NfL levels. This would be in line with recent analysis demonstrating no or just minimal change of CSF and highly related serum neurofilament concentrations over the course of disease [7, 11, 13]. Especially at the lower end of CSF NfL levels in ALS, there might be a continuous turnover of neurofilaments as a result of an equilibrium between neurodegeneration and -regeneration, with the latter depending on individual variables [e.g., genetics, resilience, exposure to environmental (epigenetic) factors throughout life [43]]. Due to such individual circumstances a certain ALS subgroup abandons that equilibrium (supposedly at an already preclinical state or rather suddenly indicating the onset of irreversible neuroaxonal damage [5, 65]), passing the threshold for disease development and CSF NfL increase which in turn relates to shorter survival. This model especially holds true for the ALS conversion of asymptomatic familial cases revealing normal CSF NfL at presymptomatic but highly elevated levels at symptomatic disease stages [4, 11, 65]. We, however, refrained from comparing NfL between sporadic and familial ALS, because of the small number of genetic variants. Impact of CSF NfL on long-term prognosis remains significant after the adjustment for several disease-modifying variables, replicating previous findings [4, 7] and suggesting NfL to aid as an independent prognostic biomarker.
The association of the same biomarker with both UMND ALS and worse prognosis is somewhat puzzling, as the UMND phenotype compared to classic ALS usually has a slower functional decline. Our results may thereby point to the existence of distinct groups displaying high CSF NfL: UMND ALS with longer survival despite high CSF NfL and ALS patients with combined UMN and LMN pathology (classic disease phenotype), high CSF NfL and worse prognosis [4] (see follow-up analysis in the Supplemental and Supplemental Fig. 3). Further studies are indeed needed to disentangle the existence of such subgroups.
The strength of our study is the availability of a considerable set of locally well-established imaging biomarkers used to understand the co-variance patterns between CSF NfL, CSF tau measures and further measures of PNS and CNS neuroaxonal damage in ALS. Limitations comprise: (1) our cross-sectional and retrospective approach, (2) the relatively small sample size of distinct clinical ALS subgroups (especially of UMND ALS, making up only 11% of our patient cohort), which kept us from performing extensive phenotype-wise analysis (of e.g., the relationship between CSF measures and neuroaxonal injury markers), as well as (3) the absence of measures of serum NfL and phosphorylated neurofilament heavy chain (pNFH) (e.g., to investigate the superiority of one marker to reflect disease severity and biomarker neuroaxonal injury). An additional limitation of the study is the heterogeneity among the number of patients who underwent each assessment (Supplemental Fig. 1).
Nevertheless our findings overall strengthen the idea that combining CSF NfL, the ptau/ttau ratio, CST DTI metrics and clinical measures (of e.g., UMN pathology) improve the diagnostic accuracy and prognostic assessment in ALS.
References
Meeter LH, Kaat LD, Rohrer JD, van Swieten JC (2017) Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol 13(7):406–419. https://doi.org/10.1038/nrneurol.2017.75
Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, Blennow K, Hansson O (2016) Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol Med 8(10):1184–1196. https://doi.org/10.15252/emmm.201606540
Kaiserova M, Grambalova Z, Otruba P, Stejskal D, Prikrylova Vranova H, Mares J, Mensikova K, Kanovsky P (2017) Cerebrospinal fluid levels of chromogranin A and phosphorylated neurofilament heavy chain are elevated in amyotrophic lateral sclerosis. Acta Neurol Scand. https://doi.org/10.1111/ane.12735
Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, Toffanin E, Cagnin A, Briani C, Pegoraro E, Sorarù G (2017) Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA Neurol 74(5):525–532. https://doi.org/10.1001/jamaneurol.2016.5398
Poesen K, Schaepdryver M de, Stubendorff B, Gille B, Muckova P, Wendler S, Prell T, Ringer TM, Rhode H, Stevens O, Claeys KG, Couwelier G, D’Hondt A, Lamaire N, Tilkin P, van Reijen D, Gourmaud S, Fedtke N, Heiling B, Rumpel M, Rödiger A, Gunkel A, Witte OW, Paquet C, Vandenberghe R, Grosskreutz J, van Damme P (2017) Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88(24):2302–2309. https://doi.org/10.1212/WNL.0000000000004029
Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM, Arnim CAF von, Böhm S, Kassubek J, Kubisch C, Lulé D, Müller H-P, Muche R, Pinkhardt E, Oeckl P, Rosenbohm A, Anderl-Straub S, Volk AE, Weydt P, Ludolph AC, Otto M (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87(1):12–20. https://doi.org/10.1136/jnnp-2015-311387
Lu C-H, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, Giovannoni G, Fratta P, Sidle K, Fish M, Orrell R, Howard R, Talbot K, Greensmith L, Kuhle J, Turner MR, Malaspina A (2015) Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84(22):2247–2257. https://doi.org/10.1212/WNL.0000000000001642
Steinacker P, Verde F, Fang L, Feneberg E, Oeckl P, Roeber S, Anderl-Straub S, Danek A, Diehl-Schmid J, Fassbender K, Fliessbach K, Foerstl H, Giese A, Jahn H, Kassubek J, Kornhuber J, Landwehrmeyer GB, Lauer M, Pinkhardt EH, Prudlo J, Rosenbohm A, Schneider A, Schroeter ML, Tumani H, Arnim CAF von, Weishaupt J, Weydt P, Ludolph AC, Yilmazer Hanke D, Otto M (2017) Chitotriosidase (CHIT1) is increased in microglia and macrophages in spinal cord of amyotrophic lateral sclerosis and cerebrospinal fluid levels correlate with disease severity and progression. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2017-317138
Schaepdryver M de, Jeromin A, Gille B, Claeys KG, Herbst V, Brix B, van Damme P, Poesen K (2017) Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2017-316605
Chen X, Chen Y, Wei Q, Ou R, Cao B, Zhao B, Shang H-F (2016) Assessment of a multiple biomarker panel for diagnosis of amyotrophic lateral sclerosis. BMC Neurol 16:173. https://doi.org/10.1186/s12883-016-0689-x
Gendron TF, Daughrity LM, Heckman MG, Diehl NN, Wuu J, Miller TM, Pastor P, Trojanowski JQ, Grossman M, Berry JD, Hu WT, Ratti A, Benatar M, Silani V, Glass JD, Floeter MK, Jeromin A, Boylan KB, Petrucelli L (2017) Phosphorylated neurofilament heavy chain: a biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann Neurol. https://doi.org/10.1002/ana.24980
Brettschneider J, Petzold A, Süssmuth SD, Ludolph AC, Tumani H (2006) Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66(6):852–856. https://doi.org/10.1212/01.wnl.0000203120.85850.54
Steinacker P, Huss A, Mayer B, Grehl T, Grosskreutz J, Borck G, Kuhle J, Lulé D, Meyer T, Oeckl P, Petri S, Weishaupt J, Ludolph AC, Otto M (2017) Diagnostic and prognostic significance of neurofilament light chain NF-L, but not progranulin and S100B, in the course of amyotrophic lateral sclerosis: data from the German MND-net. Amyotroph Later Scler Frontotemporal Degener 18(1–2):112–119. https://doi.org/10.1080/21678421.2016.1241279
Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, van Damme P, Gray E, Grosskreutz J, Jardel C, Kuhle J, Koerner S, Lamari F, Amador MDM, Mayer B, Morelli C, Muckova P, Petri S, Poesen K, Raaphorst J, Salachas F, Silani V, Stubendorff B, Turner MR, Verbeek MM, Weishaupt JH, Weydt P, Ludolph AC, Otto M (2018) Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 90(1):e22–e30. https://doi.org/10.1212/WNL.0000000000004761
Boylan KB, Glass JD, Crook JE, Yang C, Thomas CS, Desaro P, Johnston A, Overstreet K, Kelly C, Polak M, Shaw G (2013) Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 84(4):467–472
Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A, Turner MR (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2(7):748–755
Turner MR, Gray E (2016) Are neurofilaments heading for the ALS clinic? J Neurol Neurosurg Psychiatry 87(1):3–4. https://doi.org/10.1136/jnnp-2015-311934
Jiménez-Jiménez FJ, Hernánz A, Medina-Acebrón S, Bustos F de, Zurdo JM, Alonso H, Puertas I, Barcenilla B, Sayed Y, Cabrera-Valdivia F (2005) Tau protein concentrations in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neurol Scand 111(2):114–117. https://doi.org/10.1111/j.1600-0404.2005.00370.x
Paladino P, Valentino F, Piccoli T, Piccoli F, La Bella V (2009) Cerebrospinal fluid tau protein is not a biological marker in amyotrophic lateral sclerosis. Eur J Neurol 16(2):257–261. https://doi.org/10.1111/j.1468-1331.2008.02405.x
Ganesalingam J, An J, Shaw CE, Shaw G, Lacomis D, Bowser R (2011) Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J Neurochem 117(3):528–537. https://doi.org/10.1111/j.1471-4159.2011.07224.x
Süssmuth SD, Sperfeld AD, Hinz A, Brettschneider J, Endruhn S, Ludolph AC, Tumani H (2010) CSF glial markers correlate with survival in amyotrophic lateral sclerosis. Neurology 74(12):982–987. https://doi.org/10.1212/WNL.0b013e3181d5dc3b
Sussmuth SD, Tumani H, Ecker D, Ludolph AC (2003) Amyotrophic lateral sclerosis: disease stage related changes of tau protein and S100 beta in cerebrospinal fluid and creatine kinase in serum. Neurosci Lett 353(1):57–60
Grossman M, Elman L, McCluskey L, McMillan CT, Boller A, Powers J, Rascovsky K, Hu W, Shaw L, Irwin DJ, Lee VM-Y, Trojanowski JQ (2014) Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol 71(4):442–448. https://doi.org/10.1001/jamaneurol.2013.6064
Wilke C, Deuschle C, Rattay TW, Maetzler W, Synofzik M (2015) Total tau is increased, but phosphorylated tau not decreased, in cerebrospinal fluid in amyotrophic lateral sclerosis. Neurobiol Aging 36(2):1072–1074. https://doi.org/10.1016/j.neurobiolaging.2014.10.019
Bourbouli M, Rentzos M, Bougea A, Zouvelou V, Constantinides VC, Zaganas I, Evdokimidis I, Kapaki E, Paraskevas GP (2017) Cerebrospinal fluid TAR DNA-binding protein 43 combined with tau proteins as a candidate biomarker for amyotrophic lateral sclerosis and frontotemporal dementia spectrum disorders. Dement Geriatr Cogn Disord 44(3–4):144–152. https://doi.org/10.1159/000478979
Abdelhak A, Junker A, Brettschneider J, Kassubek J, Ludolph AC, Otto M, Tumani H (2015) Brain-specific cytoskeletal damage markers in cerebrospinal fluid: is there a common pattern between amyotrophic lateral sclerosis and primary progressive multiple sclerosis? Int J Mol Sci 16(8):17565–17588. https://doi.org/10.3390/ijms160817565
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Later Scler Other Motor Neuron Disord 1(5):293–299
Woo JH, Wang S, Melhem ER, Gee JC, Cucchiara A, McCluskey L, Elman L (2014) Linear associations between clinically assessed upper motor neuron disease and diffusion tensor imaging metrics in amyotrophic lateral sclerosis. PLoS One 9(8):e105753. https://doi.org/10.1371/journal.pone.0105753
Schreiber S, Abdulla S, Debska-Vielhaber G, Machts J, Dannhardt-Stieger V, Feistner H, Oldag A, Goertler M, Petri S, Kollewe K, Kropf S, Schreiber F, Heinze HJ, Dengler R, Nestor PJ, Vielhaber S (2015) Peripheral nerve ultrasound in amyotrophic lateral sclerosis phenotypes. Muscle Nerve 51(5):669–675
Chio A, Calvo A, Moglia C, Mazzini L, Mora G (2011) Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 82(7):740–746
Shibuya K, Simon NG, Geevasinga N, Menon P, Howells J, Park SB, Huynh W, Noto Y-I, Vucic S, Kiernan MC (2017) The evolution of motor cortical dysfunction in amyotrophic lateral sclerosis. Clin Neurophysiol 128(6):1075–1082. https://doi.org/10.1016/j.clinph.2017.03.004
Jin X, Jiang J-Y, Lu F-Z, Xia X-L, Wang L-X, Zheng C-J (2014) Electrophysiological differences between Hirayama disease, amyotrophic lateral sclerosis and cervical spondylotic amyotrophy. BMC Musculoskelet Disord 15:349. https://doi.org/10.1186/1471-2474-15-349
Nodera H, Takamatsu N, Shimatani Y, Mori A, Sato K, Oda M, Terasawa Y, Izumi Y, Kaji R (2014) Thinning of cervical nerve roots and peripheral nerves in ALS as measured by sonography. Clin Neurophysiol 125(9):1906–1911
Menke RA, Korner S, Filippini N, Douaud G, Knight S, Talbot K, Turner MR (2014) Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain 137(Pt 9):2546–2555
Machts J, Loewe K, Kaufmann J, Jakubiczka S, Abdulla S, Petri S, Dengler R, Heinze HJ, Vielhaber S, Schoenfeld MA, Bede P (2015) Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology 85(15):1301–1309
Albuquerque M de, Branco LMT, Rezende TJR, Andrade HMT de, Nucci A, França MC (2017) Longitudinal evaluation of cerebral and spinal cord damage in Amyotrophic Lateral Sclerosis. Neuroimage Clin 14:269–276. https://doi.org/10.1016/j.nicl.2017.01.024
Walhout R, Westeneng H-J, Verstraete E, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH (2015) Cortical thickness in ALS: towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry 86(3):288–294. https://doi.org/10.1136/jnnp-2013-306839
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR 149(2):351–356
Shen D, Cui L, Fang J, Cui B, Li D, Tai H (2016) Voxel-wise meta-analysis of gray matter changes in amyotrophic lateral sclerosis. Front Aging Neurosci 8:64
Husson F, Le S, Pages J (2011) Exploratory multivariate analysis by example using R. CRC, Boca Raton
Cardenas-Blanco A, Machts J, Acosta-Cabronero J, Kaufmann J, Abdulla S, Kollewe K, Petri S, Schreiber S, Heinze HJ, Dengler R, Vielhaber S, Nestor PJ (2016) Structural and diffusion imaging versus clinical assessment to monitor amyotrophic lateral sclerosis. Neuroimage Clin 11:408–414
Al Chalabi A, Hardiman O (2013) The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 9(11):617–628
Oberstadt M, Claßen J, Arendt T, Holzer M (2017) TDP-43 and cytoskeletal proteins in ALS. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0543-1
Petzold A (2005) Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 233(1–2):183–198. https://doi.org/10.1016/j.jns.2005.03.015
Eisen A, Weber M (2001) The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve 24(4):564–573
Yuan A, Sershen H, Basavarajappa BS, Kumar A, Hashim A, Berg M, Lee J-H, Sato Y, Rao MV, Mohan PS, Dyakin V, Julien J-P, Lee VM-Y, Nixon RA (2015) Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry 20(8):986–994. https://doi.org/10.1038/mp.2015.45
Zetterberg H, Skillbäck T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K (2016) Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol 73(1):60–67. https://doi.org/10.1001/jamaneurol.2015.3037
Jonsson M, Zetterberg H, van Straaten E, Lind K, Syversen S, Edman A, Blennow K, Rosengren L, Pantoni L, Inzitari D, Wallin A (2010) Cerebrospinal fluid biomarkers of white matter lesions—cross-sectional results from the LADIS study. Eur J Neurol 17(3):377–382. https://doi.org/10.1111/j.1468-1331.2009.02808.x
Sjögren M, Blomberg M, Jonsson M, Wahlund LO, Edman A, Lind K, Rosengren L, Blennow K, Wallin A (2001) Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J Neurosci Res 66(3):510–516. https://doi.org/10.1002/jnr.1242
Brureau A, Blanchard-Bregeon V, Pech C, Hamon S, Chaillou P, Guillemot J-C, Barneoud P, Bertrand P, Pradier L, Rooney T, Schussler N (2017) NF-L in cerebrospinal fluid and serum is a biomarker of neuronal damage in an inducible mouse model of neurodegeneration. Neurobiol Dis 104:73–84. https://doi.org/10.1016/j.nbd.2017.04.007
Ballatore C, Lee VM-Y, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8(9):663–672. https://doi.org/10.1038/nrn2194
Trojanowski JQ, Schuck T, Schmidt ML, Lee VM (1989) Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem 37(2):209–215. https://doi.org/10.1177/37.2.2492045
Blennow K, Zetterberg H, Fagan AM (2012) Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med 2(9):a006221. https://doi.org/10.1101/cshperspect.a006221
Zetterberg H (2017) Review: tau in biofluids—relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol 43(3):194–199. https://doi.org/10.1111/nan.12378
Meeter LHH, Vijverberg EG, Del Campo M, Rozemuller AJM, Donker Kaat L, Jong FJ de, van der Flier WM, Teunissen CE, van Swieten JC, Pijnenburg YAL (2018) Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology 90(14):e1231–e1239. https://doi.org/10.1212/WNL.0000000000005261
Pijnenburg YAL, Verwey NA, van der Flier WM, Scheltens P, Teunissen CE (2015) Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement (Amst) 1(4):505–512. https://doi.org/10.1016/j.dadm.2015.11.001
Menke RAL, Abraham I, Thiel CS, Filippini N, Knight S, Talbot K, Turner MR (2012) Fractional anisotropy in the posterior limb of the internal capsule and prognosis in amyotrophic lateral sclerosis. Arch Neurol 69(11):1493–1499. https://doi.org/10.1001/archneurol.2012.1122
Borsodi F, Culea V, Langkammer C, Khalil M, Pirpamer L, Quasthoff S, Enzinger C, Schmidt R, Fazekas F, Ropele S (2017) Multimodal assessment of white matter tracts in amyotrophic lateral sclerosis. PLoS One 12(6):e0178371. https://doi.org/10.1371/journal.pone.0178371
Rosenbohm A, Müller H-P, Hübers A, Ludolph AC, Kassubek J (2016) Corticoefferent pathways in pure lower motor neuron disease: a diffusion tensor imaging study. J Neurol 263(12):2430–2437. https://doi.org/10.1007/s00415-016-8281-2
Acosta-Cabronero J, Nestor PJ (2014) Diffusion tensor imaging in Alzheimer’s disease: insights into the limbic-diencephalic network and methodological considerations. Front Aging Neurosci 6:266. https://doi.org/10.3389/fnagi.2014.00266
Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A (2018) Understanding the physiopathology behind axial and radial diffusivity changes—what do we know? Front Neurol 9:92. https://doi.org/10.3389/fneur.2018.00092
Wheeler-Kingshott CAM, Cercignani M (2009) About “axial” and “radial” diffusivities. Magn Reson Med 61(5):1255–1260. https://doi.org/10.1002/mrm.21965
Toft MH, Gredal O, Pakkenberg B (2005) The size distribution of neurons in the motor cortex in amyotrophic lateral sclerosis. J Anat 207(4):399–407. https://doi.org/10.1111/j.1469-7580.2005.00465.x
Weydt P, Oeckl P, Huss A, Müller K, Volk AE, Kuhle J, Knehr A, Andersen PM, Prudlo J, Steinacker P, Weishaupt JH, Ludolph AC, Otto M (2016) Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol 79(1):152–158. https://doi.org/10.1002/ana.24552
Acknowledgements
We thank Christa Sobetzko, Department of Neurology, Otto-von-Guericke University, Magdeburg, Germany, for data collection.
Author information
Authors and Affiliations
Contributions
SS: data analysis, data interpretation, study concept and design, and drafting the manuscript. NS, FS: data analysis, data interpretation, study concept and design, and critical revision of the manuscript for important intellectual content. JAC, JK, JM, GDV, CG, DB, NH, RD, SP: acquisition of data and critical revision of the manuscript for important intellectual content. PJN: critical revision of the manuscript for important intellectual content. SV: acquisition of data, data interpretation, study concept and design, drafting the manuscript, critical revision of the manuscript for important intellectual content, and study supervision. SS had full access to all of the data of the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analysis conducted by SS, MD, NS, PhD, and FS, Otto-von-Guericke University and DZNE Magdeburg.
Funding
This work was supported by a grant to SS from the Otto-von-Guericke University Magdeburg (Grant for young scientists 2016) and by a grant to SS and SV from the Foundation of Medical Research, Frankfurt/Main, Germany.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the Stefanie Schreiber states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schreiber, S., Spotorno, N., Schreiber, F. et al. Significance of CSF NfL and tau in ALS. J Neurol 265, 2633–2645 (2018). https://doi.org/10.1007/s00415-018-9043-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9043-0