Abstract
Introduction
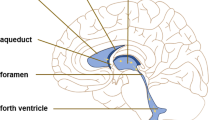
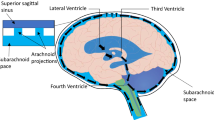
Glymphatic fluid circulation may be considered the lymphatic system of the brain and the main role of such system seems to be played by aquaporins (AQPs), a family of proteins which regulates water exchange, in particular AQP4 and 1. Alterations of glymphatic fluid circulation through AQPs variations are now emerging as central elements in the pathophysiology of different brain conditions, like hydrocephalus. This systematic review provides an insight about the role of AQPs in hydrocephalus establishment and compensation, investigating their possible role as diagnostic tools or therapeutic targets.
Methods
PubMed database was screened searching for the relevant existing literature in English language published until February 29th 2020, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
Results
A total of 40 articles met the inclusion criteria for our systematic analysis. AQP4 resulted the most studied water channel, followed by AQP1. The changes in cerebrospinal fluid (CSF), brain parenchyma and choroid plexus (CP) in different hydrocephalus type were analyzed. Moreover, important pharmacological interactions regarding AQP and molecules or conditions were discussed. A very interesting result is the general consensus on increase of AQP4 in hydrocephalic patients, unless in patients suffering from idiopathic normal pressure hydrocephalus, where AQP4 shows a tendency in reduction.
Conclusion
AQP seem to play a central role in the pathophysiology of hydrocephalus and in its compensation mechanisms. Further studies are required to definitively establish their precise roles and their quantitative changes to allow their utilization as diagnostic tools or therapeutic targets.


Similar content being viewed by others
Availability of data and material
All data and materials support published claims and comply with field standards.
Code availability
Not applicable.
References
Iliff JJ, Goldman SA, Nedergaard M (2015) Implications of the discovery of brain lymphatic pathways. Lancet Neurol 14:977–979. https://doi.org/10.1016/S1474-4422(15)00221-5
Iliff JJ, Wang M, Liao Y et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid. Sci Transl Med 4:147111. https://doi.org/10.1126/scitranslmed.3003748
Zeppenfeld DM, Simon M, Haswell JD et al (2017) Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol 74:91. https://doi.org/10.1001/jamaneurol.2016.4370
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17:1016–1024. https://doi.org/10.1016/S1474-4422(18)30318-1
Jessen NA, Munk ASF, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner’s guide. Neurochem Res 40:2583–2599. https://doi.org/10.1007/s11064-015-1581-6
Farb R, Rovira À (2020) Hydrocephalus and CSF disorders. In: Hodler J, Kubik-Huch RA, von Schulthess GK (eds) Diseases of the brain, head and neck, spine 2020–2023: diagnostic imaging. Springer, Cham (CH)
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14:265–277. https://doi.org/10.1038/nrn3468
Nakada T, Kwee IL (2019) Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 25:155–166. https://doi.org/10.1177/1073858418775027
Iliff JJ, Lee H, Yu M et al (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123:1299–1309. https://doi.org/10.1172/JCI67677
Mestre H, Hablitz LM, Xavier AL et al (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 7:e40070. https://doi.org/10.7554/eLife.40070
Guo J, Mi X, Zhan R et al (2018) Aquaporin 4 silencing aggravates hydrocephalus induced by injection of autologous blood in rats. Med Sci Monit 24:4204–4212. https://doi.org/10.12659/MSM.906936
Feng X, Papadopoulos MC, Liu J et al (2009) Sporadic obstructive hydrocephalus in Aqp4 null mice. J Neurosci Res 87:1150–1155. https://doi.org/10.1002/jnr.21927
Iliff J, Simon M (2019) CrossTalk proposal: The glymphatic system supports convective exchange of cerebrospinal fluid and brain interstitial fluid that is mediated by perivascular aquaporin-4. J Physiol (Lond) 597:4417–4419. https://doi.org/10.1113/JP277635
Raneri F, Zella MAS, Di Cristofori A et al (2017) Supplementary tests in idiopathic normal pressure hydrocephalus: a single-center experience with a combined lumbar infusion test and tap test. World Neurosurg 100:567–574. https://doi.org/10.1016/j.wneu.2017.01.003
Locatelli M, Draghi R, Cristofori ADI et al (2014) Third ventriculostomy in late-onset idiopathic aqueductal stenosis treatment: a focus on clinical presentation and radiological diagnosis. Neurol Med Chir (Tokyo) 54:1014–1021. https://doi.org/10.2176/nmc.oa.2013-0367
Wikkelso C, Hellstrom P, Klinge PM et al (2013) The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF Tap Test in patients with idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 84:562–568. https://doi.org/10.1136/jnnp-2012-303314
Marmarou A, Bergsneider M, Klinge P et al (2005) The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 57:S2-17–S2-28. https://doi.org/10.1227/01.NEU.0000168184.01002.60
Rizwan Siddiqui M, Attar F, Mohanty V et al (2018) Erythropoietin-mediated activation of aquaporin-4 channel for the treatment of experimental hydrocephalus. Childs Nerv Syst 34:2195–2202. https://doi.org/10.1007/s00381-018-3865-z
Ding Y, Zhang T, Wu G et al (2019) Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage. Exp Neurol 320:113003. https://doi.org/10.1016/j.expneurol.2019.113003
Paul L, Madan M, Rammling M et al (2009) The altered expression of aquaporin 1 and 4 in choroid plexus of congenital hydrocephalus. Fluids Barriers CNS 6:S7. https://doi.org/10.1186/1743-8454-6-S1-S7
Supuran CT (2015) Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev Neurother 15:851–856. https://doi.org/10.1586/14737175.2015.1066675
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Eide PK, Hansson H-A (2020) Blood-brain barrier leakage of blood proteins in idiopathic normal pressure hydrocephalus. Brain Res 1727:146547. https://doi.org/10.1016/j.brainres.2019.146547
Close LN, Zanaty M, Kirby P, Dlouhy BJ (2019) Acute hydrocephalus resulting from neuromyelitis optica: a case report and review of the literature. World Neurosurg 129:367–371. https://doi.org/10.1016/j.wneu.2019.05.177
Arighi A, Di Cristofori A, Fenoglio C et al (2019) Cerebrospinal fluid level of aquaporin4: a new window on glymphatic system involvement in neurodegenerative disease? JAD 69:663–669. https://doi.org/10.3233/JAD-190119
Castañeyra-Ruiz L, Hernández-Abad LG, Carmona-Calero EM et al (2019) AQP1 overexpression in the CSF of obstructive hydrocephalus and inversion of its polarity in the choroid plexus of a Chiari malformation type II case. J Neuropathol Exp Neurol 78:641–647. https://doi.org/10.1093/jnen/nlz033
Hamamoto Filho PT, Fogaroli MO, Oliveira MAC et al (2019) A rat model of neurocysticercosis-induced hydrocephalus: chronic progressive hydrocephalus with mild clinical impairment. World Neurosurg 132:e535–e544. https://doi.org/10.1016/j.wneu.2019.08.085
Long C-Y, Huang G-Q, Du Q et al (2019) The dynamic expression of aquaporins 1 and 4 in rats with hydrocephalus induced by subarachnoid haemorrhage. FN 57:182–195. https://doi.org/10.5114/fn.2019.86296
Hasan-Olive MM, Enger R, Hansson H-A et al (2019) Pathological mitochondria in neurons and perivascular astrocytic endfeet of idiopathic normal pressure hydrocephalus patients. Fluids Barriers CNS 16:39. https://doi.org/10.1186/s12987-019-0160-7
Hasan-Olive MM, Enger R, Hansson H-A et al (2019) Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia 67:91–100. https://doi.org/10.1002/glia.23528
Trillo-Contreras JL, Ramírez-Lorca R, Hiraldo-González L et al (2018) Combined effects of aquaporin-4 and hypoxia produce age-related hydrocephalus. Biochimica et Biophysica Acta (BBA) Mol Basis Dis 1864:3515–3526. https://doi.org/10.1016/j.bbadis.2018.08.006
Jeon T, Park K-S, Park S-H et al (2017) Expression of aquaporin 1 and 4 in the choroid plexus and brain parenchyma of kaolin-induced hydrocephalic rats. Korean J Neurotrauma 13:68. https://doi.org/10.13004/kjnt.2017.13.2.68
Eide PK, Hansson H-A (2017) Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol Appl Neurobiol 44:474–490. https://doi.org/10.1111/nan.12420
Limbrick DD, Baksh B, Morgan CD et al (2017) Cerebrospinal fluid biomarkers of infantile congenital hydrocephalus. PLoS ONE 12:e0172353. https://doi.org/10.1371/journal.pone.0172353
Guo Y, Weigand SD, Popescu BF et al (2017) Pathogenic implications of cerebrospinal fluid barrier pathology in neuromyelitis optica. Acta Neuropathol 133:597–612. https://doi.org/10.1007/s00401-017-1682-1
Schmidt MJ, Rummel C, Hauer J et al (2016) Increased CSF aquaporin-4, and interleukin-6 levels in dogs with idiopathic communicating internal hydrocephalus and a decrease after ventriculo-peritoneal shunting. Fluids Barriers CNS 13:12. https://doi.org/10.1186/s12987-016-0034-1
Gu W, Li F, Zhang PJ (2016) Expression and significance of aquaporin protein in Sprague–Dawley rats after experimental intraventricular hemorrhage. Cell Mol Biol 62:59–62. https://doi.org/10.14715/cmb/2016.62.4.11
Castañeyra-Ruiz L, González-Marrero I, Carmona-Calero EM et al (2016) Cerebrospinal fluid levels of tumor necrosis factor alpha and aquaporin 1 in patients with mild cognitive impairment and idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg 146:76–81. https://doi.org/10.1016/j.clineuro.2016.04.025
Ortega E, Muñoz RI, Luza N et al (2016) The value of early and comprehensive diagnoses in a human fetus with hydrocephalus and progressive obliteration of the aqueduct of Sylvius: case report. BMC Neurol 16:45. https://doi.org/10.1186/s12883-016-0566-7
Blasco E, Martorell J, De la Fuente C, Pumarola M (2014) Immunohistochemical study of aquaporins in an African Grey Parrot (Psittacus erithacus) with hydrocephalus. J Avian Med Surg 28:309–315. https://doi.org/10.1647/2013-059
Sveinsdottir S, Gram M, Cinthio M et al (2014) Altered expression of aquaporin 1 and 5 in the choroid plexus following preterm intraventricular hemorrhage. Dev Neurosci 36:542–551. https://doi.org/10.1159/000366058
Castañeyra-Ruiz L, González-Marrero I, González-Toledo JM et al (2013) Aquaporin-4 expression in the cerebrospinal fluid in congenital human hydrocephalus. Fluids Barriers CNS 10:18. https://doi.org/10.1186/2045-8118-10-18
Skjolding AD, Holst AV, Broholm H et al (2013) Differences in distribution and regulation of astrocytic aquaporin-4 in human and rat hydrocephalic brain: AQP4 in human and rat hydrocephalic brain. Neuropathol Appl Neurobiol 39:179–191. https://doi.org/10.1111/j.1365-2990.2012.01275.x
Roales-Buján R, Páez P, Guerra M et al (2012) Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathol 124:531–546. https://doi.org/10.1007/s00401-012-0992-6
Aghayev K, Bal E, Rahimli T et al (2012) Aquaporin-4 expression is not elevated in mild hydrocephalus. Acta Neurochir 154:753–759. https://doi.org/10.1007/s00701-011-1241-9
Paul L, Madan M, Rammling M et al (2011) Expression of aquaporin 1 and 4 in a congenital hydrocephalus rat model. Neurosurgery 68:462–473. https://doi.org/10.1227/NEU.0b013e3182011860
Wang D, Nykanen M, Yang N et al (2011) Altered cellular localization of aquaporin-1 in experimental hydrocephalus in mice and reduced ventriculomegaly in aquaporin-1 deficiency. Mol Cell Neurosci 46:318–324. https://doi.org/10.1016/j.mcn.2010.10.003
Skjolding AD, Rowland IJ, Søgaard LV et al (2010) Hydrocephalus induces dynamic spatiotemporal regulation of aquaporin-4 expression in the rat brain. Fluids Barriers CNS 7:20. https://doi.org/10.1186/1743-8454-7-20
Tourdias T, Dragonu I, Fushimi Y et al (2009) Aquaporin 4 correlates with apparent diffusion coefficient and hydrocephalus severity in the rat brain: a combined MRI—histological study. NeuroImage 47:659–666. https://doi.org/10.1016/j.neuroimage.2009.04.070
Smith ZA, Moftakhar P, Malkasian D et al (2007) Choroid plexus hyperplasia: surgical treatment and immunohistochemical results: case report. J Neurosurg Pediatr 107:255–262. https://doi.org/10.3171/PED-07/09/255
Shen XQ, Miyajima M, Ogino I, Arai H (2006) Expression of the water-channel protein aquaporin 4 in the H-Tx rat: possible compensatory role in spontaneously arrested hydrocephalus. J Neurosurg Pediatr 105:459–464. https://doi.org/10.3171/ped.2006.105.6.459
Mao X, Enno TL, Del Bigio MR (2006) Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci 23:2929–2936. https://doi.org/10.1111/j.1460-9568.2006.04829.x
Longatti P, Basaldella L, Orvieto E et al (2006) Aquaporin(s) expression in choroid plexus tumours. Pediatr Neurosurg 42:228–233. https://doi.org/10.1159/000092359
Bloch O, Auguste KI, Manley GT, Verkman A (2006) Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab 26:1527–1537. https://doi.org/10.1038/sj.jcbfm.9600306
Oshio K, Watanabe H, Song Y et al (2005) Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J 19:76–78. https://doi.org/10.1096/fj.04-1711fje
Clardy SL, Lucchinetti CF, Krecke KN et al (2014) Hydrocephalus in neuromyelitis optica. Neurology 82:1841–1843. https://doi.org/10.1212/WNL.0000000000000428
Gratton S, Mora C (2013) Unexplained hydrocephalus in a patient with neuromyelitis optica. In: Proceedings of the 2012 North American Neuro-Ophthalmology Society, San Antonio, TX, USA
Eide PK, Eidsvaag VA, Nagelhus EA, Hansson H-A (2016) Cortical astrogliosis and increased perivascular aquaporin-4 in idiopathic intracranial hypertension. Brain Res 1644:161–175. https://doi.org/10.1016/j.brainres.2016.05.024
Leinonen V, Koivisto AM, Savolainen S et al (2010) Amyloid and tau proteins in cortical brain biopsy and Alzheimer’s disease. Ann Neurol 68:446–453. https://doi.org/10.1002/ana.22100
Golomb J, Wisoff J, Miller DC et al (2000) Alzheimer’s disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry 68:778–781. https://doi.org/10.1136/jnnp.68.6.778
Tarasoff-Conway JM, Carare RO, Osorio RS et al (2015) Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol 11:457–470. https://doi.org/10.1038/nrneurol.2015.119
Kress BT, Iliff JJ, Xia M et al (2014) Impairment of paravascular clearance pathways in the aging brain: paravascular clearance. Ann Neurol 76:845–861. https://doi.org/10.1002/ana.24271
Reeves BC, Karimy JK, Kundishora AJ et al (2020) Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med 26:285–295. https://doi.org/10.1016/j.molmed.2019.11.008
Bateman GA (2013) Hypertensive slit ventricle syndrome: pseudotumor cerebri with a malfunctioning shunt? JNS 119:1503–1510. https://doi.org/10.3171/2013.7.JNS13390
Hamilton K, Koueik J, Maganti R, Iskandar B (2019) Slit ventricle syndrome leads to 10-year history of repetitive transient central herniation masquerading as seizures: hydrocephalus case report. World Neurosurg 126:134–138. https://doi.org/10.1016/j.wneu.2019.02.106
Sivaganesan A, Krishnamurthy R, Sahni D, Viswanathan C (2012) Neuroimaging of ventriculoperitoneal shunt complications in children. Pediatr Radiol 42:1029–1046. https://doi.org/10.1007/s00247-012-2410-6
Del Bigio MR, Di Curzio DL (2016) Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids Barriers CNS. https://doi.org/10.1186/s12987-016-0025-2
Funding
The authors declare that they have no sources of funding.
Author information
Authors and Affiliations
Contributions
Concept of the article: AC, CL. Literature search and data analysis: CL, AC. Draft of the article: AC, CL, AA. Critically revision of the article: all authors.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest/competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Integrity of research and reporting
The manuscript does not contain clinical studies or patient data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Laurentis, C., Cristaldi, P., Arighi, A. et al. Role of aquaporins in hydrocephalus: what do we know and where do we stand? A systematic review. J Neurol 268, 4078–4094 (2021). https://doi.org/10.1007/s00415-020-10122-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10122-z