Abstract
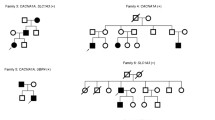
Variants in CACNA1A are classically related to episodic ataxia type 2, familial hemiplegic migraine type 1, and spinocerebellar ataxia type 6. Over the years, CACNA1A has been associated with a broader spectrum of phenotypes. Targeted analysis and unbiased sequencing of CACNA1A result not only in clear molecular diagnoses, but also in large numbers of variants of uncertain significance (VUS), or likely pathogenic variants with a phenotype that does not directly match the CACNA1A spectrum. Over the last years, targeted and clinical exome sequencing in our center has identified 41 CACNA1A variants. Ultimately, variants were considered pathogenic or likely pathogenic in 23 cases, with most phenotypes ranging from episodic or progressive ataxia to more complex ataxia syndromes, as well as intellectual disability and epilepsy. In two cases, the causality of the variant was discarded based on non-segregation or an alternative diagnosis. In the remaining 16 cases, the variant was classified as uncertain, due to lack of opportunities for segregation analysis or uncertain association with a non-classic phenotype. Phenotypic variability and the large number of VUS make CACNA1A a challenging gene for neurogenetic diagnostics. Accessible functional read-outs are clearly needed, especially in cases with a non-classic phenotype.

Similar content being viewed by others
Availability of data and material
The protocol and analysis pipeline are available upon request. Anonymized patient data are not available for ethical reasons.
Code availability
Not applicable.
References
Llinás R, Sugimori M, Lin JW, Cherksey B (1989) Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci USA 86:1689–1693
Turner TJ, Adams ME, Dunlap K (1992) Calcium channels coupled to glutamate release identified by omega-Aga-IVA. Science 258:310–313
Uchitel OD, Protti DA, Sanchez V, Cherksey BD, Sugimori M, Llinás R (1992) P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci USA 89:3330–3333
Lübbert M, Goral RO, Keine C et al (2019) Ca(V)2.1 α(1) subunit expression regulates presynaptic Ca(V)2.1 abundance and synaptic strength at a central synapse. Neuron 101:260-273.e266
Choi K-D, Choi J-H (2016) Episodic ataxias: clinical and genetic features. J Mov Disord 9:129–135
Nachbauer W, Nocker M, Karner E et al (2014) Episodic ataxia type 2: phenotype characteristics of a novel CACNA1A mutation and review of the literature. J Neurol 261:983–991
Guterman EL, Yurgionas B, Nelson AB (2016) Pearls & oysters: episodic ataxia type 2: case report and review of the literature. Neurology 86:e239–e241
Riant F, Mourtada R, Saugier-Veber P, Tournier-Lasserve E (2008) Large CACNA1A deletion in a family with episodic ataxia type 2. Arch Neurol 65:817–820
Labrum RW, Rajakulendran S, Graves TD et al (2009) Large scale calcium channel gene rearrangements in episodic ataxia and hemiplegic migraine: implications for diagnostic testing. J Med Genet 46:786–791
Riant F, Lescoat C, Vahedi K et al (2010) Identification of CACNA1A large deletions in four patients with episodic ataxia. Neurogenetics 11:101–106
Wan J, Mamsa H, Johnston JL et al (2011) Large genomic deletions in CACNA1A cause episodic ataxia type 2. Front Neurol 2:51
Pietrobon D (2010) CaV2.1 channelopathies. Pflugers Arch 460:375–393
Tyagi S, Ribera AB, Bannister RA (2019) Zebrafish as a model system for the study of severe CaV2.1 (alpha1A) channelopathies. Front Mol Neurosci 12:329
Guida S, Trettel F, Pagnutti S et al (2001) Complete loss of P/Q calcium channel activity caused by a CACNA1A missense mutation carried by patients with episodic ataxia type 2. Am J Hum Genet 68:759–764
Kors EE, Terwindt GM, Vermeulen FL et al (2001) Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol 49:753–760
Tottene A, Fellin T, Pagnutti S et al (2002) Familial hemiplegic migraine mutations increase Ca(2+) influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc Natl Acad Sci USA 99:13284–13289
Ikeuchi T, Takano H, Koide R et al (1997) Spinocerebellar ataxia type 6: CAG repeat expansion in alpha1A voltage-dependent calcium channel gene and clinical variations in Japanese population. Ann Neurol 42:879–884
Matsuyama Z, Kawakami H, Maruyama H et al (1997) Molecular features of the CAG repeats of spinocerebellar ataxia 6 (SCA6). Hum Mol Genet 6:1283–1287
Riess O, Schöls L, Bottger H et al (1997) SCA6 is caused by moderate CAG expansion in the alpha1A-voltage-dependent calcium channel gene. Hum Mol Genet 6:1289–1293
Zhuchenko O, Bailey J, Bonnen P et al (1997) Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet 15:62–69
García-Planells J, Cuesta A, Vilchez JJ, Martínez F, Prieto F, Palau F (1999) Genetics of the SCA6 gene in a large family segregating an autosomal dominant “pure” cerebellar ataxia. J Med Genet 36:148–151
Mariotti C, Gellera C, Grisoli M, Mineri R, Castucci A, Di Donato S (2001) Pathogenic effect of an intermediate-size SCA-6 allele (CAG)(19) in a homozygous patient. Neurology 57:1502–1504
Rentiya Z, Hutnik R, Mekkam YQ, Bae J (2020) The pathophysiology and clinical manifestations of spinocerebellar ataxia type 6. The Cerebellum 19:459–464
Globas C, du Montcel ST, Baliko L et al (2008) Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov Disord 23:2232–2238
Travaglini L, Nardella M, Bellacchio E et al (2017) Missense mutations of CACNA1A are a frequent cause of autosomal dominant nonprogressive congenital ataxia. Eur J Paediatr Neurol 21:450–456
Blumkin L, Michelson M, Leshinsky-Silver E, Kivity S, Lev D, Lerman-Sagie T (2010) Congenital ataxia, mental retardation, and dyskinesia associated with a novel CACNA1A mutation. J Child Neurol 25:892–897
García Segarra N, Gautschi I, Mittaz-Crettol L et al (2014) Congenital ataxia and hemiplegic migraine with cerebral edema associated with a novel gain of function mutation in the calcium channel CACNA1A. J Neurol Sci 342:69–78
Myers Candace T, McMahon Jacinta M, Schneider Amy L et al (2016) De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am J Hum Genet 99:287–298
Jiang X, Raju PK, D’Avanzo N et al (2019) Both gain-of-function and loss-of-function de novo CACNA1A mutations cause severe developmental epileptic encephalopathies in the spectrum of Lennox-Gastaut syndrome. Epilepsia 60:1881–1894
Hayashida T, Saito Y, Ishii A et al (2018) CACNA1A-related early-onset encephalopathy with myoclonic epilepsy: a case report. Brain Dev 40:130–133
Reinson K, Oiglane-Shlik E, Talvik I et al (2016) Biallelic CACNA1A mutations cause early onset epileptic encephalopathy with progressive cerebral, cerebellar, and optic nerve atrophy. Am J Med Genet A 170:2173–2176
Verriello L, Pauletto G, Nilo A et al (2021) Epilepsy and episodic ataxia type 2: family study and review of the literature. J Neurol 268:4296–4302
Le Roux M, Barth M, Gueden S et al (2021) CACNA1A-associated epilepsy: electroclinical findings and treatment response on seizures in 18 patients. Eur J Paediatr Neurol 33:75–85
Imbrici P, Jaffe SL, Eunson LH et al (2004) Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain 127:2682–2692
Indelicato E, Unterberger I, Nachbauer W et al (2021) The electrophysiological footprint of CACNA1A disorders. J Neurol 268:2493–2505
Rajakulendran S, Graves TD, Labrum RW et al (2010) Genetic and functional characterisation of the P/Q calcium channel in episodic ataxia with epilepsy. J Physiol 588:1905–1913
Gelfand AA (2018) Episodic syndromes of childhood associated with migraine. Curr Opin Neurol 31:281–285
Blumkin L, Leshinsky-Silver E, Michelson M et al (2015) Paroxysmal tonic upward gaze as a presentation of de-novo mutations in CACNA1A. Eur J Paediatr Neurol 19:292–297
Fernández-Alvarez E (2018) Transient benign paroxysmal movement disorders in infancy. Eur J Paediatr Neurol 22:230–237
Giffin NJ, Benton S, Goadsby PJ (2002) Benign paroxysmal torticollis of infancy: four new cases and linkage to CACNA1A mutation. Dev Med Child Neurol 44:490–493
Vila-Pueyo M, Sintas C, Flotats M et al (2013) A loss-of-function CACNA1A mutation causing benign paroxysmal torticollis of infancy. J Headache Pain 14:P24
Cuenca-Leon E, Corominas R, Fernandez-Castillo N et al (2008) Genetic analysis of 27 Spanish patients with hemiplegic migraine, basilar-type migraine and childhood periodic syndromes. Cephalalgia 28:1039–1047
Roubertie A, Echenne B, Leydet J et al (2008) Benign paroxysmal tonicupgaze, benign paroxysmal torticollis, episodic ataxia and CACNA1A mutation in a family. J Neurol 255:1600–1602
Damaj L, Lupien-Meilleur A, Lortie A et al (2015) CACNA1A haploinsufficiency causes cognitive impairment, autism and epileptic encephalopathy with mild cerebellar symptoms. Eur J Hum Genet 23:1505–1512
Indelicato E, Nachbauer W, Karner E et al (2019) The neuropsychiatric phenotype in CACNA1A mutations: a retrospective single center study and review of the literature. Eur J Neurol 26:66-e67
Agathe R, Véronique H, Florence R et al (2017) Cognitive disorders in patients with CACNA1A mutations. Eur J Paediatr Neurol 21:e22
Humbertclaude V, Krams B, Nogue E et al (2018) Benign paroxysmal torticollis, benign paroxysmal vertigo, and benign tonic upward gaze are not benign disorders. Dev Med Child Neurol 60:1256–1263
Humbertclaude V, Riant F, Krams B et al (2020) Cognitive impairment in children with CACNA1A mutations. Dev Med Child Neurol 62:330–337
Neveling K, Feenstra I, Gilissen C et al (2013) A post-hoc comparison of the utility of sanger sequencing and exome sequencing for the diagnosis of heterogeneous diseases. Hum Mutat 34:1721–1726
Lek M, Karczewski KJ, Minikel EV et al (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536:285–291
Pfundt R, Del Rosario M, Vissers L et al (2017) Detection of clinically relevant copy-number variants by exome sequencing in a large cohort of genetic disorders. Genet Med 19:667–675
Krumm N, Sudmant PH, Ko A et al (2012) Copy number variation detection and genotyping from exome sequence data. Genome Res 22:1525–1532
Maas RP, Schieving JH, Schouten M, Kamsteeg EJ, van de Warrenburg BP (2016) The genetic homogeneity of capos syndrome: four new patients with the c.2452G>A (p.Glu818Lys) mutation in the ATP1A3 gene. Pediatr Neurol 59:71-75.e71
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Pradotto L, Mencarelli M, Bigoni M, Milesi A, Di Blasio A, Mauro A (2016) Episodic ataxia and SCA6 within the same family due to the D302N CACNA1A gene mutation. J Neurol Sci 371:81–84
Angelini C, Van Gils J, Bigourdan A et al (2019) Major intra-familial phenotypic heterogeneity and incomplete penetrance due to a CACNA1A pathogenic variant. Eur J Med Genet 62:103530
Nardello R, Plicato G, Mangano GD et al (2020) Two distinct phenotypes, hemiplegic migraine and episodic Ataxia type 2, caused by a novel common CACNA1A variant. BMC Neurol 20:155
Indelicato E, Boesch S (2021) From genotype to phenotype: expanding the clinical spectrum of CACNA1A variants in the era of next generation sequencing. Front Neurol 12:639994
Denier C, Ducros A, Vahedi K et al (1999) High prevalence of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology 52:1816
Jen J, Wan J, Graves M et al (2001) Loss-of-function EA2 mutations are associated with impaired neuromuscular transmission. Neurology 57:1843–1848
Spacey SD, Hildebrand ME, Materek LA, Bird TD, Snutch TP (2004) Functional implications of a novel EA2 mutation in the P/Q-type calcium channel. Ann Neurol 56:213–220
Wan J, Khanna R, Sandusky M, Papazian DM, Jen JC, Baloh RW (2005) CACNA1A mutations causing episodic and progressive ataxia alter channel trafficking and kinetics. Neurology 64:2090–2097
Kraus RL, Sinnegger MJ, Glossmann H, Hering S, Striessnig J (1998) Familial hemiplegic migraine mutations change alpha1A Ca2+ channel kinetics. J Biol Chem 273:5586–5590
Hans M, Luvisetto S, Williams ME et al (1999) Functional consequences of mutations in the human alpha1A calcium channel subunit linked to familial hemiplegic migraine. J Neurosci 19:1610–1619
Kraus RL, Sinnegger MJ, Koschak A et al (2000) Three new familial hemiplegic migraine mutants affect P/Q-type Ca(2+) channel kinetics. J Biol Chem 275:9239–9243
Ducros A, Denier C, Joutel A et al (2001) The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med 345:17–24
Melliti K, Grabner M, Seabrook GR (2003) The familial hemiplegic migraine mutation R192Q reduces G-protein-mediated inhibition of P/Q-type (Ca(V)2.1) calcium channels expressed in human embryonic kidney cells. J Physiol 546:337–347
Müllner C, Broos LA, van den Maagdenberg AM, Striessnig J (2004) Familial hemiplegic migraine type 1 mutations K1336E, W1684R, and V1696I alter Cav2.1 Ca2+ channel gating: evidence for beta-subunit isoform-specific effects. J Biol Chem 279:51844–51850
Tottene A, Pivotto F, Fellin T, Cesetti T, van den Maagdenberg AM, Pietrobon D (2005) Specific kinetic alterations of human CaV2.1 calcium channels produced by mutation S218L causing familial hemiplegic migraine and delayed cerebral edema and coma after minor head trauma. J Biol Chem 280:17678–17686
Weiss N, Sandoval A, Felix R, Van den Maagdenberg A, De Waard M (2008) The S218L familial hemiplegic migraine mutation promotes deinhibition of Ca(v)2.1 calcium channels during direct G-protein regulation. Pflugers Arch 457:315–326
Adams PJ, Garcia E, David LS, Mulatz KJ, Spacey SD, Snutch TP (2009) Ca(V)2.1 P/Q-type calcium channel alternative splicing affects the functional impact of familial hemiplegic migraine mutations: implications for calcium channelopathies. Channels (Austin) 3:110–121
Serra SA, Fernàndez-Castillo N, Macaya A, Cormand B, Valverde MA, Fernández-Fernández JM (2009) The hemiplegic migraine-associated Y1245C mutation in CACNA1A results in a gain of channel function due to its effect on the voltage sensor and G-protein-mediated inhibition. Pflugers Arch 458:489–502
Stam AH, Luijckx GJ, Poll-The BT et al (2009) Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. J Neurol Neurosurg Psychiatry 80:1125–1129
Zangaladze A, Asadi-Pooya AA, Ashkenazi A, Sperling MR (2010) Sporadic hemiplegic migraine and epilepsy associated with CACNA1A gene mutation. Epilepsy Behav 17:293–295
Russell MB, Ducros A (2011) Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol 10:457–470
Brusich DJ, Spring AM, James TD, Yeates CJ, Helms TH, Frank CA (2018) Drosophila CaV2 channels harboring human migraine mutations cause synapse hyperexcitability that can be suppressed by inhibition of a Ca2+ store release pathway. PLoS Genet 14:e1007577
Zutt R, Elting JW, Santens P, Luijckx GJR, Tijssen MAJ (2020) Two cases with postural axial tremor: consider a genetic origin. Parkinsonism Relat Disord 77:152–154
Molloy A, Kimmich O, Martindale J, Moore H, Hutchinson M, O’Riordan S (2013) A novel CACNA1A mutation associated with adult-onset, paroxysmal head tremor. Mov Disord 28:842–843
Duque KR, Marsili L, Sturchio A et al (2021) Progressive ataxia with hemiplegic migraines: a phenotype of CACNA1A missense mutations, not CAG repeat expansions. Cerebellum 20:134–139
Reetz K, Costa AS, Mirzazade S et al (2013) Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6. Brain 136:905–917
Schulz JB, Borkert J, Wolf S et al (2010) Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage 49:158–168
Naik S, Pohl K, Malik M, Siddiqui A, Josifova D (2011) Early-onset cerebellar atrophy associated with mutation in the CACNA1A gene. Pediatr Neurol 45:328–330
Tantsis EM, Gill D, Griffiths L et al (2016) Eye movement disorders are an early manifestation of CACNA1A mutations in children. Dev Med Child Neurol 58:639–644
Quade A, Thiel A, Kurth I et al (2020) Paroxysmal tonic upgaze: a heterogeneous clinical condition responsive to carbonic anhydrase inhibition. Eur J Paediatr Neurol 25:181–186
Karczewski KJ, Francioli LC, Tiao G et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. bioRxiv. 531210.
Acknowledgements
We thank several colleagues from the departments of neurology, pediatric neurology, and human genetics of Radboud university medical center, Nijmegen, the Netherlands for their role in collecting relevant patient data. We would like to thank C. Gilissen (department of human genetics, Radboud university medical center, Nijmegen, the Netherlands) for collecting information on the chance of de novo mutations in CACNA1A.
Funding
M.P. Hommersom, T.H. van Prooije, M. Pennings, M.I. Schouten, H. van Bokhoven and E-J. Kamsteeg report no disclosures. M.P. Hommersom is supported by a grant from the Radboud university medical center and Donders Institute for Brain, Cognition and Behaviour. T.H. van Prooije is supported by a grant from ZonMW. H. van Bokhoven is supported by grants from Radboud university medical center. B. van de Warrenburg is supported by grants from Radboud university medical center, ZonMW, Hersenstichting, uniQure, Gossweiler Foundation, the Michael J Fox Foundation, and has served on scientific advisory boards of uniQure and Vico Therapeutics. Authors of this publication are members of the European Reference Network for Rare Neurological Diseases—Project ID No 739510.
Author information
Authors and Affiliations
Contributions
MPH, THP and BPCW conceptualized the study. MPH and THP drafted the manuscript. MP had a major role in data acquisition. MIS and E-JK interpreted the data. HB, E-JK and BPCW revised the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
Clinical exome sequencing was approved by the Medical Review Ethics Committee, Region Arnhem–Nijmegen, Number 2011/188.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
Hommersom, M.P., van Prooije, T.H., Pennings, M. et al. The complexities of CACNA1A in clinical neurogenetics. J Neurol 269, 3094–3108 (2022). https://doi.org/10.1007/s00415-021-10897-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10897-9