Abstract
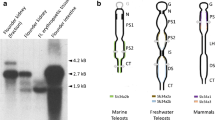
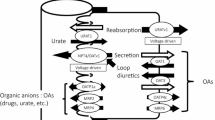
The aim of this study was to elucidate mechanisms of Pi handling in toads (Bufo bufo). We introduced toads to experimental solutions of various [Pi] and high Pi diets and measured urine and lymph [Pi]. Both lymph and urine [Pi] increased with increasing Pi loads, indicating Pi absorption across skin and intestine. An initial fragment of a NaPi-II type transporter was amplified from kidney, and the full-length sequence was obtained. The protein showed the molecular hallmarks of NaPi-IIb transporters. When expressed in Xenopus oocytes the clone showed unusual pH dependence, but apparent affinity constants for Pi and Na+ were in the range of other NaPi-II transporters. Expression profiling showed that the transporter was present in skin, intestine and kidney. Reverse transcription–polymerase chain reaction assays on dissected renal tubules indicated expression in the collecting duct system. Collecting tubules and ducts were isolated, perfused and microelectrode recordings showed electrogenic Pi transport in apical and basolateral membranes. Taken together, our results show that Pi is handled by intestine, kidney and skin. The presently cloned NaPi-IIb is a likely candidate involved in Pi absorption across these epithelia. In addition, electrophysiological experiments suggest that the collecting duct system plays an important role in Pi homeostasis.









Similar content being viewed by others
References
Baginski ES, Foa PP, Zak B (1967) Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem 13(4):326–332
Bacconi A, Virkki LV, Biber J, Murer H, Forster IC (2005) Renouncing electroneutrality is not free of charge: switching on electrogenicity in a Na+-coupled phosphate cotransporter. P N A S USA 102(35):12606–12611
Beyenbach KW (2004) Kidneys sans glomeruli. Am J Physiol Renal Physiol 286:F811–F827
Biber J, Gisler SM, Hernando N, Wagner CA, Murer H (2004) PDZ interactions and proximal tubular phosphate reabsorption. Am J Physiol Renal Physiol 287:F871–F875
Bijvoet OLM, Reitsma PJ (1977) Phylogeny of renal phosphate transport in the vertebrates. In: Massry SG, Ritz E (eds) Advances in experimental medicine and biology: phosphate metabolism. Plenum, New York, pp 41–53
Boutilier RG, Stiffler DF, Toews DP (1992) Exchange of respiratory gases, ions, and water in amphibious and aquatic amphibians. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians. The University of Chicago Press, Chicago, pp 81–124
Collins JF, Bai L, Ghishan FK (2004) The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflugers Arch 447:647–652
Custer M, Meier F, Schlatter E, Greger R, Garcia-Perez A, Biber J, Murer H (1993) Localization of NaPi-1, a Na-Pi cotransporter, in rabbit kidney proximal tubules. I. mRNA localization by reverse transcription/polymerase chain reaction. Pflugers Arch 424(3–4):203–209
Dantzler WH (1992) Comparative aspects of renal function. In: Seldin DW, Giebisch G (eds) The kidney: physiology and pathophysiology, 2nd edn. Raven, New York, pp 885–942
Elger M, Werner A, Herter P, Kohl B, Kinne RKH, Hentschel H (1998) Na–Pi cotransport sites in the proximal tubule and collecting tubule of winter flounder (Pleuronectes americanus). Am J Physiol Renal Physiol 274:F374–F383
Forster IC, Kohler K, Biber J, Murer H (2002) Forging the link between structure and function of electrogenic cotransporters: the renal type IIa Na+/Pi cotransporter as a case study. Prog Biophys Mol Biol 80(3):69–108
Graham C, Nalbant P, Schölermann B, Hentschel H, Kinne RKH, Werner A (2003) Characterization of a type IIb sodium-phosphate cotransporter from zebrafish (Danio rerio) kidney. Am J Physiol Renal Physiol 284(4):F727–F736
Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J (1998) Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci USA 95(24):14564–14569
Hogben CAM, Bollman JL (1951) Excretion of phosphate by isolated frog kidney. Am J Physiol 164:662–669
Ishizuya-Oka A, Stolow MA, Ueda S, Shi Y-B (1997) Temporal and spatial expression of an intestinal \({{\text{Na}}^{{\text{ + }}} } \mathord{\left/ {\vphantom {{{\text{Na}}^{{\text{ + }}} } {{\text{PO}}}}} \right. \kern-\nulldelimiterspace} {{\text{PO}}}^{{3 - }}_{4} \) cotransporter correlates with epithelial transformation during thyroid hormone-dependent frog metamorphosis. Dev Genet 20:53–66
Jensen LJ, Willumsen NJ, Amstrup J, Larsen EH (2003) Proton pump-driven cutaneous chloride uptake in anuran amphibian. Biochim Biophys Acta 1618(2):120–132
Jørgensen BC (1997) 200 years of amphibian water economy: from Robert Townson to the present. Biol Rev Camb Philos Soc 72(2):153–237
Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT (2002) Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflugers Arch 445(1):139–146
Kohl B, Herter P, Hülseweh B, Elger M, Hentschel H, Kinne RKH, Werner A (1996) Na–Pi cotransport in flounder: same transport system in kidney and intestine. Am J Physiol Renal Fluid Electrolyte Physiol 270:F937–F944
Larsen EH (1988) NaCl transport in amphibian skin. In: Greger R (ed) Advances in comparative and environmental physiology, vol 1. Springer, Berlin Heidelberg New York, pp 189–248
Møbjerg N, Larsen EH, Jespersen Å (1998) Morphology of the nephron in the mesonefros of Bufo bufo (Amphibia, Anura, Bufonidae). Acta Zool 79:31–50
Møbjerg N, Larsen EH, Novak I (2002) K+ transport in the mesonephric collecting duct system of the toad, Bufo bufo. Microelectrode recordings from isolated and perfused tubules. J Exp Biol 205:897–904
Møbjerg N, Larsen EH, Novak I (2004) Ion transport mechanisms in the mesonephric collecting duct system of the toad Bufo bufo, as revealed by microelectrode recordings from isolated and perfused tubules. Comp Biochem Physiol Part A Mol Integr Physiol 137/3:585–595
Møbjerg N, Jespersen Å, Wilkinson M (2004) Morphology of the nephron in the mesonephros of Geotrypetes seraphini (Amphibia, Gymnophiona, Caeciliaidae). J Morphol 262(2):583–607
Murer H, Hernando N, Forster I, Biber J (2000) Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80(4):1373–1409
Murer H, Forster I, Biber J (2004) The sodium phosphate cotransporter family SLC34. Pflügers Arch 447:763–767
Nalbant P, Boehmer C, Dehmelt L, Wehner F, Werner A (1999) Functional characterization of a Na+-phosphate cotransporter (NaPi-II) from zebrafish and identification of related transcripts. J Physiol 520(1):79–89
Reimer RJ, Edwards RH (2004) Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflügers Arch 447:629–635
Renfro JL (1995) Solute transport by flounder renal cells in primary culture. In: Wood CM, Shuttleworth TJ (eds) Cellular and molecular approaches to fish ionic regulation. Academic, New York, pp 147–171
Renfro JL (1999) Recent developments in teleost renal transport. J Exp Zool 283:653–661
Shoemaker VH, Hillman SS, Hillyard SD, Jackson DC, McClanahan LL, Withers PC, Wygoda ML (1992) Exchange of water, ions, and respiratory gases in terrestrial amphibians. In: Feder ME, Warren WB (eds) Environmental physiology of the amphibians. The University of Chicago Press, Chicago, pp 125–150
Uchiyama M, Konno N (2006) Hormonal regulation of ion and water transport in anuran amphibians. Gen Comp Endocrinol 147:54–61
Walker AM, Hudson CJ (1936) The role of the tubule in the excretion of inorganic phosphate by the amphibian kidney. Am J Physiol 118:167–173
Werner A, Moore ML, Mantei N, Biber J, Semenza G, Murer H (1991) Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci USA 88:9608–9612
Werner A, Murer H, Kinne RK (1994) Cloning and expression of a renal Na–Pi cotransport system from flounder. Am J Physiol Renal Physiol 267:311–317
Werner A, Kinne RK (2001) Evolution of the Na–Pi cotransport systems. Am J Physiol Regul Integr Comp Physiol 280(2):R301–R312
Acknowledgement
We thank Mai-Britt Andersen, Sofie Nehammer and Rikke Thorsteinsson, who were undergraduate students in our laboratory and performed spectrophotometrical Pi determinations. Dr. Jan Amstrup is warmly thanked for his help with RNA isolations and primer constructions and Dr. Aslak Jørgensen for providing DNA sequence facilities at the DBL–Institute for Health Research and Development, Charlottenlund, Denmark. We would also like to express our thanks to Dr. Berit Kristensen for introduction to Pi determination methods, Prof. Dr. Erik Hviid Larsen for helpful discussions and Ms. Alice Scheuer for taking care of the toads. Ms. Anni Olsen, Ms. Birthe Petersen, Mr. Thomas Sørensen and Mr. Arne Nielsen are kindly thanked for technical assistance.
The project was supported by the Carlsberg Foundation, the Danish Natural Science Research Council and Wellcome Trust (UK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Møbjerg, N., Werner, A., Hansen, S.M. et al. Physiological and molecular mechanisms of inorganic phosphate handling in the toad Bufo bufo . Pflugers Arch - Eur J Physiol 454, 101–113 (2007). https://doi.org/10.1007/s00424-006-0176-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0176-0