Abstract
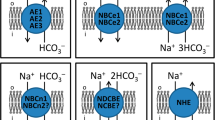
Metabolic and biochemical changes during breast carcinogenesis enhance cellular acid production. Extrusion of the acid load from the cancer cells raises intracellular pH, while it decreases extracellular pH creating an inverted pH gradient across the plasma membrane compared to normal cells and promoting cancer cell metabolism, proliferation, migration, and invasion. We investigated the effects of breast carcinogenesis on the mechanisms of cellular pH control using multicellular epithelial organoids freshly isolated from human primary breast carcinomas and matched normal breast tissue. Intracellular pH was measured by fluorescence microscopy, while protein expression was investigated by immunofluorescence imaging and immunoblotting. We found that cellular net acid extrusion increased during human breast carcinogenesis due to enhanced Na+,HCO3 −-cotransport, which created an alkaline shift (~0.3 units of magnitude) in steady-state intracellular pH of human primary breast carcinomas compared to normal breast tissue. Na+/H+-exchange activity and steady-state intracellular pH in the absence of CO2/HCO3 − were practically unaffected by breast carcinogenesis. These effects were evident under both acidic (pH 6.8, representative of the tumor microenvironment) and physiological (pH 7.4) extracellular conditions. Protein expression of the Na+,HCO3 −-cotransporter NBCn1 (SLC4A7), which has been linked to breast cancer susceptibility in multiple genome-wide association studies, was twofold higher in human breast carcinomas compared to matched normal breast tissue. Protein expression of the Na+/H+-exchanger NHE1 (SLC9A1) was markedly less affected. We propose that upregulated NBCn1 during human breast carcinogenesis contributes to the characteristic acid distribution within human breast carcinomas and thereby plays a pathophysiological role for breast cancer development and progression.





Similar content being viewed by others
References
Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R, Morrison J, Maranian M, Pooley KA, Luben R, Eccles D et al (2009) Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41:585–590
Aronson PS, Suhm MA, Nee J (1983) Interaction of external H+ with the Na+-H+ exchanger in renal microvillus membrane vesicles. J Biol Chem 258:6767–6771
Boedtkjer E, Praetorius J, Aalkjaer C (2006) NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ Res 98:515–523
Boedtkjer E, Praetorius J, Fuchtbauer EM, Aalkjaer C (2008) Antibody-independent localization of the electroneutral Na+-HCO3 − cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol 294:C591–C603
Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Füchtbauer AC, Simonsen U, Fuchtbauer EM, Aalkjaer C (2011) Disruption of Na+,HCO3 −-cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+-sensitivity and hypertension development in mice. Circulation 124:1819–1829
Boedtkjer E, Bunch L, Pedersen SF (2012) Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: similarities, differences and implications for cancer therapy. Curr Pharm Des 18:1345–1371
Boedtkjer E, Damkier HH, Aalkjaer C (2012) NHE1 knockout reduces blood pressure and arterial media/lumen ratio with no effect on resting pHi in the vascular wall. J Physiol 590:1895–1906
Boedtkjer E, Moreira JM, Mele M, Vahl P, Wielenga VT, Christiansen PM, Jensen VE, Pedersen SF, Aalkjaer C (2013) Contribution of Na+,HCO3 −-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer 132:1288–1299
Boron WF, De Weer P (1976) Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67:91–112
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95
Cardone RA, Casavola V, Reshkin SJ (2005) The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 5:786–795
Chen Y, Choong LY, Lin Q, Philp R, Wong CH, Ang BK, Tan YL, Loh MC, Hew CL, Shah N, Druker BJ et al (2007) Differential expression of novel tyrosine kinase substrates during breast cancer development. Mol Cell Proteomics 6:2072–2087
Ch’en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD (2008) S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br J Pharmacol 153:972–982
Chiche J, Le FY, Vilmen C, Frassineti F, Daniel L, Halestrap AP, Cozzone PJ, Pouyssegur J, Lutz NW (2012) In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int J Cancer 130:1511–1520
Damkier HH, Nielsen S, Praetorius J (2006) An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290:H172–H180
De Milito A, Marino ML, Fais S (2012) A rationale for the use of proton pump inhibitors as antineoplastic agents. Curr Pharm Des 18:1395–1406
Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF (2014) Regulation and roles of bicarbonate transporters in cancer. Front Physiol 5:130
Han W, Woo JH, Yu JH, Lee MJ, Moon HG, Kang D, Noh DY (2011) Common genetic variants associated with breast cancer in Korean women and differential susceptibility according to intrinsic subtype. Cancer Epidemiol Biomarkers Prev 20:793–798
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Hulikova A, Vaughan-Jones RD, Swietach P (2011) Dual role of CO2/HCO3 − buffer in the regulation of intracellular pH of three-dimensional tumor growths. J Biol Chem 286:13815–13826
Johannessen TC, Wagner M, Straume O, Bjerkvig R, Eikesdal HP (2013) Tumor vasculature: the Achilles’ heel of cancer? Expert Opin Ther Targets 17:7–20
Larsen AM, Krogsgaard-Larsen N, Lauritzen G, Olesen CW, Honoré Hansen S, Boedtkjer E, Pedersen SF, Bunch L (2012) Gram-scale solution-phase synthesis of selective sodium bicarbonate co-transport inhibitor S0859: in vitro efficacy studies in breast cancer cells. ChemMedChem 7:1808–1814
Lauritzen G, Jensen MB, Boedtkjer E, Dybboe R, Aalkjaer C, Nylandsted J, Pedersen SF (2010) NBCn1 and NHE1 expression and activity in δNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp Cell Res 316:2538–2553
Lauritzen G, Stock CM, Lemaire J, Lund SF, Jensen MF, Damsgaard B, Petersen KS, Wiwel M, Ronnov-Jessen L, Schwab A, Pedersen SF (2012) The Na+/H+ exchanger NHE1, but not the Na+,HCO3 − cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett 317:172–183
Long J, Shu XO, Cai Q, Gao YT, Zheng Y, Li G, Li C, Gu K, Wen W, Xiang YB, Lu W et al (2010) Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev 19:2357–2365
Neri D, Supuran CT (2011) Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10:767–777
Orlowski J, Grinstein S (2004) Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447:549–565
Parker MD, Boron WF (2013) The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93:803–959
Parks SK, Chiche J, Pouyssegur J (2011) pH control mechanisms of tumor survival and growth. J Cell Physiol 226:299–308
Parks SK, Chiche J, Pouyssegur J (2013) Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 13:611–623
Pedersen SF (2006) The Na+/H+ exchanger NHE1 in stress-induced signal transduction: implications for cell proliferation and cell death. Pflugers Arch 452:249–259
Petersen OW, Hoyer PE, Hilgers J, Briand P, Van Deurs B (1985) Characterization of epithelial cell islets in primary monolayer cultures of human breast carcinomas by the tetrazolium reaction for glucose 6-phosphate dehydrogenase. Virchows Arch B Cell Pathol Incl Mol Pathol 50:27–42
Ro HA, Carson JH (2004) pH microdomains in oligodendrocytes. J Biol Chem 279:37115–37123
Rotin D, Steele-Norwood D, Grinstein S, Tannock I (1989) Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res 49:205–211
Schwab A, Fabian A, Hanley PJ, Stock C (2012) Role of ion channels and transporters in cell migration. Physiol Rev 92:1865–1913
Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R (2004) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286:C1443–C1452
Silva AS, Yunes JA, Gillies RJ, Gatenby RA (2009) The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res 69:2677–2684
Stock C, Schwab A (2006) Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol (Oxford) 187:149–157
Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, Tajima K, Tanaka H, Iwata H, Iwase H, Matsuo K (2012) A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population. Breast Cancer Res Treat 132:711–721
Trivedi B, Danforth WH (1966) Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem 241:4110–4112
Acknowledgments
The authors would like to thank Dr. Ole William Petersen, University of Copenhagen, for advice on preparing epithelial organoids. We are grateful to Dr. Christian Aalkjaer, Aarhus University, and Dr. Stine F. Pedersen, University of Copenhagen, for fruitful discussions. Dr. Jeppe Praetorius, Aarhus University, is thanked for generously providing the NBCn1 antibody. This work was supported by the Danish Council for Independent Research (10-094816 to E.B.), the Novo Nordisk Foundation (to E.B.), and the Danish Cancer Society (R72-A4273-13-S2 to E.B.).
Ethical standards
The experiments in this article comply with the current Danish laws.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S., Mele, M., Vahl, P. et al. Na+,HCO3 −-cotransport is functionally upregulated during human breast carcinogenesis and required for the inverted pH gradient across the plasma membrane. Pflugers Arch - Eur J Physiol 467, 367–377 (2015). https://doi.org/10.1007/s00424-014-1524-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1524-0