Abstract
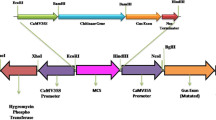
Brassica juncea BjCHI1 is a unique chitinase with two chitin-binding domains. Here, we show that, unlike other chitinases, potato-expressed BjCHI1 shows hemagglutination ability. BjCHI1 expression in B. juncea seedlings is induced by Rhizoctonia solani infection, suggesting its protective role against this fungus. To verify this, transgenic potato (Solanum tuberosum L. cv. Desiree) plants expressing BjCHI1 generated by Agrobacterium-mediated transformation were challenged with R. solani. We also transformed potato with a cDNA encoding Hevea brasiliensis β-1,3-glucanase, designated HbGLU, and a pBI121-derivative that contains cDNAs encoding both BjCHI1 and HbGLU. In vitro fungal bioassays using Trichoderma viride showed that extracts from transgenic potato lines co-expressing BjCHI1 and HbGLU inhibited fungal growth better than extracts from transgenic potato expressing either BjCHI1 or HbGLU, suggesting a synergistic effect. Consistently, in vivo fungal bioassays with soil-borne R. solani on young transgenic potato plants indicated that the co-expressing plants showed healthier root development than untransformed plants or those that expressed either BjCHI1 or HbGLU. Light microscopy and transmission electron microscopy revealed abundant intact R. solani hyphae and monilioid cells in untransformed roots and disintegrated fungus in the BjCHI1-expressing and the BjCHI1 and HbGLU co-expressing plants. Observations of collapsed epidermal cells in the co-expressing potato roots suggest that these proteins effectively degrade the fungal cell wall, producing elicitors that initiate other defense responses causing epidermal cell collapse that ultimately restricts further fungal penetration.









Similar content being viewed by others
Abbreviations
- BjCHI1 :
-
Brassica juncea chitinase 1
- CaMV :
-
Cauliflower Mosaic Virus
- HbGLU :
-
Hevea brasiliensis β-1,3-glucanase
- MS :
-
Murashige and Skoog
- PVDF :
-
Polyvinylidene difluoride
- TEM :
-
Transmission electron microscopy
- UDA :
-
Urtica dioica agglutinin
References
Abeles FB, Forrence LE (1970) Temporal and hormonal control of β-1,3-glucanase in Phaseolus vulgaris L. Plant Physiol 45:395–400
Banville GJ, Carling DE, Otrysko BE (1999) Rhizoctonia disease on potato. In: Sneh B, Jabaji-Hare S, Neate S, Dijst G (eds) Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Kluwer, Dordrecht, pp 321–330
Benhamou N, Theriault G (1992) Treatment with chitosan enhances resistance of tomato plants to the crown and root rot pathogen, Fusarium oxysporum f. sp. radicis-lycopersici. Physiol Mol Plant Pathol 41:33–52
Benhamou N, Broglie K, Chet I, Broglie R (1993) Cytology of infection of 35S-bean chitinase transgenic canola plants by Rhizoctonia solani: cytochemical aspects of chitin breakdown in vivo. Plant J 4:295–305
Benhamou N, Lafontaine PJ, Nicole M (1994) Seed treatment with chitosan induces systemic resistance to Fusarium crown and root rot in tomato plants. Phytopathology 84:1432–1444
Boller T (1985) Induction of hydrolases as a defense reaction against pathogens. In: Key JL, Kosuge T (eds) Cellular and molecular biology of plant stress. Liss, New York, pp 247–262
Boller T (1992) Biochemical analysis of chitinase and β-1,3-glucanses. In: Gurr SJ, McPherson MJ, Bowles DJ (eds) Molecular plant pathology: a practical approach, vol II. IRL Press, Oxford, pp 23–30
Boller T, Gehri A, Mauch F, Vogeli U (1983) Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157:22–31
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Broglie K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254:1194–1197
Chrispeels MJ, Raikhel NV (1991) Lectins, lectin genes, and their role in plant defense. Plant Cell 3:1–9
Chye ML, Cheung KY (1995) β-1,3-glucanase is highly-expressed in laticifers of Hevea brasiliensis. Plant Mol Biol 29:397–402
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Dietze J, Blau A, Willmitzer L (1995) Agrobacterium-mediated transformation of potato (Solanum tuberosum). In: Potrykus I, Spangenberg G (eds) Gene transfer to plants. Cold Spring Harbor Laboratory Press, New York, pp 24–29
Does M, Houterman PM, Dekker HL, Cornelissen BJ (1999) Processing, targeting and antifungal activity of stinging nettle agglutinin in transgenic tobacco. Plant Physiol 120:421–431
Foster RC, Rovira AD, Cock TW (1983) Ultrastructure of the root-soil interface. The American Phytopathogical Society, St Paul, MN
Fung KL, Zhao KJ, He ZM, Chye ML (2002) Brassica juncea chitinase BjCHI1 binds chitin and shows antifungal activity in vitro. Plant Mol Biol 50:283–294
Hadwinger LA, Chiang C, Victory S, Horovitz D (1988) The molecular biology of chitosan in plant–pathogen interactions and its application to agriculture. In: Skjak G, Anthonsen B, Sandford P (eds) Chitin and chitosan: sources, chemistry, biochemistry, physical properties and applications. Elsevier, Amsterdam, pp 119–138
Isaac S (1992) Fungal–plant interactions. Chapman and Hall, London
Iseli B, Boller T, Neuhaus J-M (1993) The N-terminal cysteine-rich domain of tobacco class I chitinase is essential for chitin binding but not for catalytic or antifungal activity. Plant Physiol 103:221–226
Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8:97–109
Kush A, Goyvaerts E, Chye ML, Chua NH (1990) Laticifer specific gene expression of Hevea brasiliensis (rubber tree). Proc Natl Acad Sci USA 87:1787–1790
Leuba JL, Stossel P (1986) Chitosan and other polyamines: antifungal activity and interaction with biological membranes. In: Muzzarelli R, Jeauniaux C, Gooday GW (eds) Chitin in nature and technology. Plenum, New York, pp 215–222
Lin W, Anuratha CS, Datta K, Potrykus I, Muthukrishnan S, Datta SK (1995) Genetic engineering of rice for resistance to sheath blight. Bio/Technology 13:686–691
Mauch F, Mauch-Mani B, Boller T (1988) Antifungal hydrolases in pea tissue II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol 88:936–942
Nagy F, Kay SA, Chua NH (1988) Analysis of gene expression in trangenic plants. In: Gelvin SV, Schilperoort RA (eds) Plant molecular biology manual. Kluwer, Dordrecht, pp B4:1–29
Rasmussen U, Bojsen K, Collinge DB (1992) Cloning and characterization of a pathogen-induced chitinase in Brassica napus. Plant Mol Biol 20:277–287
Roberts P (1999) Rhizoctonia-forming fungi. A taxonomic guide. Royal Botanic Gardens, Kew
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schlumbaum A, Mauch F, Vogeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324:365–367
Taylor KACC (1995) A modification of the phenol–sulfuric acid method of total sugar determination. Appl Biochem Biotechnol 53:207–214
Van Damme EJM, Barre A, Rouge P, Peumans WJ (2004) Potato lectin: an updated model of a unique chimeric plant protein. Plant J 37:34–45
Weinhold AR, Sinclair JB (1999) Rhizoctonia solani: penetration, colonization and host response. In: Sneh B, Jabaji-Hare S, Neate S, Dijst G (eds) Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Kluwer, Dordrecht, pp 163–174
Wirth SJ, Wolf GA (1990) Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. J Microbiol Methods 12:197–205
Wright LM, Van Damme EJM, Barre A, Allens AK, Van Leuven, Reynolds CD, Rouge P, Peumans W (1999) Isolation, characterization, molecular cloning and molecular modeling of two lectins of different specificities from bluebell (Scilla campanulata) bulbs. Biochem J 340:299–308
Zhao KJ, Chye ML (1999) Methyl jasmonate induces expression of a novel Brassica juncea chitinase with two chitin-binding domains. Plant Mol Biol 40:1009–1018
Zhu Q, Maher EA, Masoud S, Dixon RA, Lamb CJ (1994) Enhanced protection against fungal attack by constitutive co-expression of chitinase and glucanase genes in trangenic tobacco. Bio/Technology 12:807–812
Acknowledgments
We thank Prof. Z.P. Lin (Peking University) for wild-type potato Desiree, Prof. E.C. Pua (National University of Singapore) for B. juncea seeds, X.Z. Ouyang for technical assistance in microscopy, A. Mak for N-terminal sequencing, and W. Ho and P. Tam for helpful discussions. This project was supported by the Department of Botany (University of Hong Kong), the Biotechnology Research Institute (Hong Kong University of Science and Technology) and the Research Grants Council of Hong Kong (Plant and Fungal Biotechnology Area of Excellence). K.J.Z. (Postdoctoral Fellowship) and K.L.F. (Postgraduate Studentship) were supported by the University of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chye, ML., Zhao, KJ., He, ZM. et al. An agglutinating chitinase with two chitin-binding domains confers fungal protection in transgenic potato. Planta 220, 717–730 (2005). https://doi.org/10.1007/s00425-004-1391-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1391-6