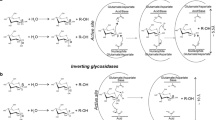
Abstract
The functions of plant glycoside hydrolases and transglycosidases have been studied using different biochemical and molecular genetic approaches. These enzymes are involved in the metabolism of various carbohydrates containing compounds present in the plant tissues. The structural and functional diversity of the carbohydrates implies a vast spectrum of enzymes involved in their metabolism. Complete genome sequence of Arabidopsis and rice has allowed the classification of glycoside hydrolases in different families based on amino acid sequence data. The genomes of these plants contain 29 families of glycoside hydrolases. This review summarizes the current research on plant glycoside hydrolases concerning their principal functional roles, which were attributed to different families. The majority of these plant glycoside hydrolases are involved in cell wall polysaccharide metabolism. Other functions include their participation in the biosynthesis and remodulation of glycans, mobilization of energy, defence, symbiosis, signalling, secondary plant metabolism and metabolism of glycolipids.




Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- AGP:
-
Arabinogalactan protein
- DBE:
-
Starch-debranching enzyme
- DIBOA:
-
2,4-Dihydroxy-1,4-benzoxazin-3-one
- DIMBOA:
-
2,4-Dihydroxy-7-methoxy -1,4-benzoxazin-3-one
- CAZY:
-
Carbohydrate-active enzymes
- Endo H:
-
Endoglucosaminidase
- ER:
-
Endoplasmatic reticulum
- IAA:
-
Indole-3-acetic acid
- GE:
-
β-Glucan elicitor
- GEBP:
-
Glucan elicitor binding protein
- GH:
-
Glycoside hydrolase
- GT:
-
Glycosyltransferase
- GUS:
-
β-d-Glucuronidase
- NF:
-
Nod factor
- PG:
-
Polygalacturonase
- PR:
-
Pathogenesis-related
- XET:
-
Xyloglucan endotransglucosylase
References
Aspeborg H, Schrader J, Coutinho PM, Stam M, Kallas A, Djerbi S, Nilsson P, Denman S, Amini B, Sterky F, Master E, Sandberg G, Mellerowicz E, Sundberg B, Henrissat B, Teeri TT (2005) Carbohydrate-active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiol 137:983–997
Atkinson RG, Schroder R, Hallet I, Cohen D, Macrae EA (2002) Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol 129:122–133
Bachmann M, Keller F (1995) Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Inter- and intracellular compartmentation. Plant Physiol 109:991–998
Beck E, Ziegler P (1989) Biosynthesis and degradation of starch in higher plants. Annu Rev Plant Physiol Plant Mol Biol 40:95–117
Beintema JJ, Terwisscha van Scheltinga AC (1996) Plant lysozymes. In: Jollès P (ed) Lysozymes: model enzymes in biochemistry and biology. Birkhäuser-Verlag, Basel, pp 75–86
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry, 5th edn. W.H. Freeman, New York
Beffa RS, Neuhaus JM, Meins F Jr (1993) Physiological compensation in antisense transformants: specific induction of an ‘ersatz’ glucan endo-1,3-β-glucosidase in plants infected with necrotizing viruses. Proc Natl Acad Sci 90:8792–8796
Beffa RS, Hoefer RM, Thomas R, Meins F (1996) Decreased susceptibility to viral disease of β 1,3-glucanase-deficient plants generated by antisense transformation. Plant Cell 8:1001–1011
Boisson M, Gomord V, Audran C, Berger N, Dubreucq B, Granier F, Lerouge P, Faye L, Caboche M, Lepiniec L (2001) Arabidopsis glucosidase I mutants reveal a critical role of N-glycan trimming in seed development. EMBO J 20:1010–1019
Boller T, Gehri A, Mauch F, Vögeli U (1983) Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157:22–31
Boller T, Kende H (1979) Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol 63:1123–1132
Boudart G, Jamet E, Rossignol M, Lafitte C, Borderies G, Jauneau A, Esquerre-Tugaye MT, Pont-Lezica R (2005) Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes: identification by mass spectrometry and bioinformatics. Proteomics 5:212–221
Boudart G, Minic Z, Albenne C, Canut H, Jamet E, Pont-Lezica R (2007) Cell wall proteome. In: Samaj S, Thelen J (eds) Plant proteomics. Springer, Berlin, pp 169–185
Bowles DJ (1990) Defense-related proteins in higher plants. Annu Rev Biochem 59:873–907
Brett CT (2000) Cellulose microfibrils in plants: biosynthesis, deposition, and integration into the cell wall. Int Rev Cytol 199:161–199
Broglie K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia Solani. Science 254:1194–1197
Brunner F, Stintzi A, Fritig B, Legrand M (1998) Substrate specificities of tobacco chitinases. Plant J 14:225–234
Brzobohaty B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K (1993) Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science 262:1051–1054
Buckeridge MS, Dietrich SMC, Lima DU (2000a) Galactomannans as the reserve carbohydrate of legume seeds. In: Gupta AK, Kaur N (eds) Developments in crop science, vol 26. Elsevier Science BV, Amsterdam, pp 283–316
Buckeridge MS, Pessoa dos Santos H, Tiné MAS (2000b) Mobilisation of storage cell wall polysaccharides in seeds. Plant Physiol Biochem 38:141–156
Burn JE, Hurley UA, Birch RJ, Arioli T, Cork A, Williamson RE (2002) The cellulose-deficient Arabidopsis mutant rsw3 is defective in a gene encoding a putative glucosidase II, an enzyme processing N-glycans during ER quality control. Plant J 32:949–960
Burton RA, Farrokhi N, Bacic A, Fincher GB (2005) Plant cell wall polysaccharide biosynthesis: real progress in the identification of participating genes. Planta 221:309–312
Carmi N, Zhang GF, Petreikov M, Gao ZF, Eyal Y, Granot D, Schaffer AA (2003) Cloning and functional expression of alkaline α-galactosidase from melon fruit: similarity to plant SIP proteins uncovers a novel family of plant glycosyl hydrolases. Plant J 33:97–106
Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47:445–476
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3:1–30
Chen M, Liu X, Wang Z, Song J, Qi Q, Wang PG (2005) Modification of plant N-glycans processing: the future of producing therapeutic protein by transgenic plants. Med Res Rev 25:343–360
Chivasa S, Ndimba BK, Simon WJ, Robertson D, Yu XL, Knox JP, Bolwell P, Slabas AR (2002) Proteomic analysis of the Arabidopsis thaliana cell wall. Electrophoresis 23:1754–1765
Chrost B, Krupinska K (2000) Genes with homologies to known α-galactosidases are expressed during senescence of barley leaves. Physiol Plant 110:111–119
Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K (1993) Plant chitinases. Plant J 3:31–40
Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol 13:171–201
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–856
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol 125:131–134
Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D (2002) The growing world of expansins. Plant Cell Physiol 43:1436–1444
Coutinho PM, Stam M, Blanc E, Henrissat B (2003) Why so many carbohydrate-active enzymes related genes in plants? Trends Plant Sci 8:563–565
Critchley JM, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26:89–100
Crombie HJ, Chengappa S, Hellyer A, Reid JSG (1998) A xyloglucan oligosaccharide-active, transglycosylating β-d-glucosidase from the cotyledons of nasturtium (Tropaeolum majus L.) seedlings—purification, properties and characterization of a cDNA clone. Plant J 15:27–38
Datta SK, Muthukrishnan S (1999) Pathogenesis-related proteins in plants. CRC, Washington, ISBN 0-8493-0697-3
del Campillo E, Bennett AB (1996) Pedicel breakstrength and cellulase gene expression during tomato flower abscission. Plant Physiol 111:813–820
Delmer DP (1999) Cellulose biosynthesis: exciting times for a difficult field of study. Annu Rev Plant Physiol Plant Mol Biol 50:245–276
Dénarié J, Debellé F, Promé JC (1996) Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu Rev Biochem 65:503–535
De Jong AJ, Cordewener J, Lo Schiavo F, Terzi M, Vandekerckhove J, Van Kammen A, De Vries SC (1992) A carrot somatic embryo mutant is rescued by chitinase. Plant Cell 4:425–433
Davies GJ, Henrissat B (2002) Plant glyco-related genomics. Structural enzymology of carbohydrate-active enzymes: implications for the post-genomic era. Biochem Soc Trans 30:291–297
Doehlert DC, Knutson CA (1991) Two classes of starch debranching enzymes from developing maize kernels. J Plant Physiol 138:566–572
Edwards M, Dea ICM, Bulpin PV, Reid JSG (1985) Xyloglucan (amyloid) mobilisation in the cotyledons of Tropaeolum majus L. seeds following germination. Planta 163:133–140
Escamilla-Trevino LL, Chen W, Card ML, Shih MC, Cheng CL, Poulton JE (2006) Arabidopsis thaliana beta-glucosidases BGLU45 and BGLU46 hydrolyse monolignol glucosides. Phytochemistry 67:1651–1660
Fenwick GR, Heaney RK, Mullin WJ (1983) Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr 18:123–201
Fincher GB (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40:305–346
Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist 161:641–675
Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ (1992) Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J 282:821–828
Fulton LM, Cobbett CS (2003) Two alpha-l-arabinofuranosidase genes in Arabidopsis thaliana are differentially expressed during vegetative growth and flower development. J Exp Bot 54:2467–2467
Gachon CM, Langlois-Meurinne M, Saindrenan P (2005) Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci 10:542–549
Gallant DJ, Bouchet B, Baldwin PM (1997) Microscopy of starch: evidence of a new level of granule organization. Carbohydr Polym 32:177–191
Geisler-Lee J, Geisler M, Coutinho PM, Segerman B, Nishikubo N, Takahashi J, Aspeborg H, Djerbi S, Master E, Andersson-Gunneras S, Sundberg B, Karpinski S, Teeri TT, Kleczkowski LA, Henrissat B, Mellerowicz EJ (2006) Poplar carbohydrate-active enzymes: gene identification and expression analyses. Plant Physiol 140:946–962
Geurts R, Franssen H (1996) Signal transduction in Rhizobium-induced nodule formation. Plant Physiol 112:447–453
Goddijn OJM, Smeekens S (1998) Mini-review: sensing trehalose biosynthesis in plants. Plant J 14:143–146
González-Carranza ZH, Whitelaw CA, Swarup R, Roberts JA (2002) Temporal and spatial expression of a polygalacturonase during leaf and flower abscission in oilseed rape and Arabidopsis. Plant Physiol 128:534–543
Goujon T, Minic Z, El Amrani A, Lerouxel O, Aletti E, Lapierre C, Joseleau JP, Jouanin L (2003) AtBXL1, a novel higher plant (Arabidopsis thaliana) putative beta-xylosidase gene, is involved in secondary cell wall metabolism and plant development. Plant J 33:677–690
Grison R, Grezes-Besset B, Schneider M, Lucante N, Olsen L, Leguay JJ, Toppan A (1996) Field tolerance to fungal pathogens of Brassica napus constitutively expressing a chimeric chitinase gene. Nat Biotechnol 14:643–646
Hamel F, Boivin R, Tremblay C, Bellamare G (1997) Structural and evolutionary relationships among chitinases of flowering plants. J Mol Evol 44:614–624
Hanfrey C, Fife M, Buchanan-Wollaston V (1996) Leaf senescence in Brassica napus: expression of genes encoding pathogenesis-related proteins. Plant Mol Biol 30:597–609
Harborne JB, Mabry TJ (1982) The flavonoids: advances in research. Chapman and Hall, London
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Henrissat B (1998) Glycosidase families. Biochem Soc Trans 26:153–156
Henrissat B, Coutinho PM, Davies GJ (2001) A census of carbohydrate-active enzymes in the genome of Arabidopsis thaliana. Plant Mol Biol 47:55–72
Heredia A, Jimenez A, Guillen R (1995) Composition of plant cell walls. Z Lebensm Unters Forsch 200:24–31
Hilson P, Allemeersch J, Altmann T, Aubourg S, Avon A, Beynon J, Bhalerao RP, Bitton F, Caboche M, Cannoot B, Chardakov V, Cognet-Holliger C, Colot V, Crowe M, Darimont C, Durinck S, Eickhoff H, de Longevialle AF, Farmer EE, Grant M, Kuiper MT, Lehrach H, Leon C, Leyva A, Lundeberg J, Lurin C, Moreau Y, Nietfeld W, Paz-Ares J, Reymond P, Rouze P, Sandberg G, Segura MD, Serizet C, Tabrett A, Taconnat L, Thareau V, Van Hummelen P, Vercruysse S, Vuylsteke M, Weingartner M, Weisbeek PJ, Wirta V, Wittink FR, Zabeau M, Small I (2004) Versatile gene-specific sequence tags for Arabidopsis functional genomics: transcript profiling and reverse genetics applications. Genome Res 14:2176–2189
Hong S, Sexton R, Tucker ML (2000) Analysis of gene promoters for two tomato polygalacturonases expressed in abscission zones and the stigma. Plant Physiol 123:869–881
Hsieh MC, Graham TL (2001) Partial purification and characterization of a soybean β-glucosidase with high specific activity for isoflavone conjugates. Phytochemistry 58:995–1005
Ishimizu T, Sasaki A, Okutani S, Maeda M, Yamagishi M, Hase S (2004) Endo-beta-mannosidase, a plant enzyme acting on N-glycan: purification, molecular cloning, and characterization. J Biol Chem 279:38555–38562
Jach G, Görnhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8:97–109
Jakubowska A, Kowalczyk S (2005) A specific enzyme hydrolyzing 6-O(4-O)-indole-3-ylacetyl-beta-d-glucose in immature kernels of Zea mays. J Plant Physiol 162:207–201
Jamet E, Canut H, Boudart G, Pont-Lezica RF (2006) Cell wall proteins: a new insight through proteomics. Trends Plant Sci 11:33–39
Kaushal G, Pastuszak I, Hatanaka KI, Elbein AD (1990a) Purification to homogeneity and properties of glucosidase II from mung bean seedlings and suspension-cultured soybean cells. J Biol Chem 265:16271–16279
Kaushal G, Szumilo T, Pastuszak I, Elbein AD (1990b) Purification to homogeneity and properties of mannosidase II from mung bean seedlings. Biochemistry 29:2168–2176
Kim JB, Olek AT, Carpita NC (2000) Cell wall and membrane-associated exo-beta-d-glucanases from developing maize seedlings. Plant Physiol 123:471–486
Kimura Y, Matsuo S, Tsurusaki S, Kimura M, Hara-Nishimura I, Nishimura M (2002) Subcellular localization of endo-beta-N-acetylglucosaminidase and high-mannose type free N-glycans in plant cell. Biochim Biophys Acta 1570:38–46
Kleczkowski K, Schell J (1995) Phytohormones conjugates: nature and function. Crit Rev Plant Sci 14:283–298
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed Engl 44:3358–3393
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Koes RE, Quattrocchino F, Mol JNM (1994) The flavonoid biosynthetic pathway in plants: function and evolution. BioEssays 16:123–132
Kotake T, Dina S, Konishi T, Kaneko S, Igarashi K, Samejima M, Watanabe Y, Kimura K, Tsumuraya Y (2005) Molecular cloning of a {beta}-galactosidase from radish that specifically hydrolyzes {beta}-(1->3)- and {beta}-(1->6)-galactosyl residues of Arabinogalactan protein. Plant Physiol 138:1563–1576
Kotake T, Tsuchiya K, Aohara T, Konishi T, Kaneko S, Igarashi K, Samejima M, Tsumuraya Y (2006) An alpha-l-arabinofuranosidase/beta-d-xylosidase from immature seeds of radish (Raphanus sativus L.). J Exp Bot 57:2353–2362
Krammer G, Winterhalter P, Schwab M, Shreier P (2002) Glycosidically bound aroma compounds in the fruits of Prunus species: apricot (P. armeniaca L.), peach (P. persica L.), (P. domestica L. ssp. Syriaca). Postharvest Biol Technol 39:778–781
Kwon HK, Yokoyama R, Nishitani K (2005) A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant Cell Physiol 46:843–857
Lashbrook CC (2005) New insights into cell wall disassembly during fruit ripening. Stewart Postharvest Rev 3:1–18
Lashbrook CC, Gonzalez-Bosch C, Bennett AB (1994) Two divergent endo-β-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell 6:1485–1493
Leah R, Kigel J, Svendsen I, Mundy J (1995) Biochemical and molecular characterization of a barley seed beta-glucosidase. J Biol Chem 270:15789–15797
Lee RC, Hrmova M, Burton RA, Lahnstein J, Fincher GB (2003) Bifunctional family 3 glycoside hydrolases from barley with α-l-arabinofuranosidase and β-d-xylosidase activity. J Biol Chem 278:5377–5387
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 26:1109–1120
Lee RH, Lin MC, Chen SC (2004) A novel alkaline alpha-galactosidase gene is involved in rice leaf senescence. Plant Mol Biol 55:281–295
Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Laine AC, Gomord V, Faye L (1998) N-glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol Biol 38:31–48
Leubner-Metzger G, Meins F Jr (1999) Functions and regulation of plant beta-1,3-glucanases (PR-2). In: Datta SK, Muthukrishnan S (eds) Pathogenesis-related proteins in plants. CRC, Boca Raton, pp 49–76
Leung DWM (1992) Involvement of plant chitinase in sexual reproduction of higher plants. Phytochemistry 31:1899–1900
Lim EK, Baldauf S, Li Y, Elias L, Worrall D, Spencer SP, Jackson RG, Taguchi G, Ross J, Bowles DJ (2003) Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology 13:139–145
Limpens E, Bisseling T (2003) Signaling in symbiosis. Curr Opin Plant Biol 6:343–305
Lin W, Anuratha CS, Datta K, Potrykus I, Muthukrishnan S, Datta SK (1995) Genetic engineering of rice for resistance to sheath blight. Biotechnology 13:686–691
Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, Parker J, Somerville S, Schulze-Lefert P (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Lloyd JR, Kossmann J, Ritte G (2005) Leaf starch degradation comes out of the shadows. Trends Plant Sci 10:130–137
Lotan T, Ori N, Fluhr R (1989) Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell 1:881–887
Mauch F, Mauch-Mani B, Boller T (1988) Antifungal hydrolases in pea tissue II. Inhibition of fungal growth by combinations of chitinase and beta-1,3-glucanase. Plant Physiol 88:936–942
Martin I, Dopico B, Munoz FJ, Esteban R, Oomen RJ, Driouich A, Vincken JP, Visser R, Labrador E (2005) In vivo expression of a Cicer arietinum beta-galactosidase in potato tubers leads to a reduction of the galactan side-chains in cell wall pectin. Plant Cell Physiol 46:1613–1622
Mega T (2005) Glucose trimming of N-glycan in endoplasmic reticulum is indispensable for the growth of Raphanus sativus seedling (kaiware radish). Biosci Biotechnol Biochem 69:1353–1364
Mérida A, Rodriguez-Galan JM, Vincent C, Romero JM (1999) Expression of the granule-bound starch synthase I (Waxy) gene from snapdragon is developmentally and circadian clock regulated. Plant Physiol 120:401–410
Minic Z, Brown S, De Kouchkovsky Y, Schultze M, Staehelin C (1998) Purification and characterization of a novel chitinase-lysozyme, of another chitinase, both hydrolyzing Rhizobium meliloti Nod factors, and of a pathogenesis-related protein from Medicago sativa roots. Biochem J 332:329–335
Minic Z, Do C-T, Rihouey C, Morin H, Lerouge P, Jouanin L (2006) Purification, functional characterization, cloning and identification of mutants of a seed specific arabinan hydrolase in Arabidopsis. J Exp Bot 57:2339–2351
Minic Z, Jamet E, Négroni L, der Garabedian PA, Zivy M, Jouanin L (2007) A sub-proteome of Arabidopsis thaliana mature stems trapped on Concanavalin A is enriched in cell wall glycoside hydrolase. Proteomics. J Exp Bot 58:2503–2512
Minic Z, Jouanin L (2006) Plant glycoside hydrolases involved in cell wall polysaccharides degradation. Plant Plysiol Biochem 44:435–449
Minic Z, Rihouey C, Do CT, Lerouge P, Jouanin L (2004) Purification and characterization of enzymes exhibiting beta-d-xylosidase activities in stem tissues of Arabidopsis. Plant Physiol 135:867–878
Molhoj M, Jorgensen B, Ulvskov P, Borkhardt B (2001) Two Arabidopsis thaliana genes, KOR2 and KOR3, which encode membrane-anchored endo-1,4-beta-d-glucanases, are differentially expressed in developing leaf trichomes and their support cells. Plant Mol Biol 46:263–275
Molhoj M, Pagant S, Hofte H (2002) Towards understanding the role of membrane-bound endo-beta-1,4-glucanases in cellulose biosynthesis. Plant Cell Physiol 43:1399–406
Morimoto S, Harioka T, Shoyama Y 1995 Purification and characterization of flavone-specific β-glucuronidase from callus culture of Scutellaria baicalensis Georgi. Planta 195:535–540
Morimoto S, Tateishi N, Matsuda T, Tanaka H, Taura F, Furuya N, Matsuyama N, Shoyama Y (1998) Novel hydrogen peroxide metabolism in suspension cells of Scutellaria baicalensis Georgi. J Biol Chem 273:12606–12611
Müller J, Boller T, Wiemken A (1995) Trehalose and trehalase in plants: recent developments. Plant Sci 112:1–9
Müller J, Wiemken A, Aeschbacher RA (1999) Trehalose metabolism in sugar sensing and plant development. Plant Sci 147:37–34
Muñoz JA, Coronado C, Pérez-Hormaeche J, Kondorosi A, Ratet P, Palomares AJ (1998) MsPG3, a Medicago sativa polygalacturonase gene expressed during the alfalfa–Rhizobium meliloti interaction. Proc Natl Acad Sci USA 95:9687–9692
Nagano AJ, Matsushima R, Hara-Nishimura I (2005) Activation of an ER-body-localized beta-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol 46:1140–1148
Navazio L, Baldan B, Mariani P, Gerwig GJ, Vliegenthart JFG 1996) Primary structure of the N-linked carbohydrate chains of calreticulin from spinach leaves. Glycoconj J 13:977–983
Neale AD, Wahleithner JA, Lund M, Bonnett HT, Kelly A, Meeks-Wagner DR, Peacock WJ, Dennis ES (1990) Chitinase, B-1,3-glucanase, osmotin, and extension are expressed in tobacco explants during flower formation. Plant Cell 2:673–684
Neuhaus JM, Fritig B, Linthorst HJM, Meins F, Mikkelsen JD, Ryals J (1996) A revised nomenclature for chitinase genes. Plant Mol Biol Rep 14:102–104
Neuhaus JM (1999) Plant chitinases (PR-3, PR-4, PR-8, PR-11). In: Datta SK, Muthukrishnan S (eds) Pathogenesis-related proteins in plants. CRC, Boca Raton, pp 77–105
Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Hofte H (1998) A plasma membrane-bound putative endo-1,4-beta-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J 17:5563–5576
Niemeyer HM (1988) Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones): defence chemicals in the Gramineae. Phytochemistry 27:3349–3358
Okita TW (1992) Is there an alternative pathway for starch synthesis? Plant Physiol 100:560–564
Ohmiya Y, Samejima M, Shiroishi M, Amano Y, Kanda T, Sakai F, Hayashi T (2000) Evidence that endo-1,4-beta-glucanases act on cellulose in suspension-cultured poplar cells. Plant J 24:147–158
Ohmiya Y, Takeda T, Nakamura S, Sakai F, Hayashi T (1995) Purification and properties of wall-bound endo-1,4-beta-glucanase from suspension-cultured poplar cells. Plant Cell Physiol 36:607–614
Ovtsyna AO, Dolgikh EA, Kilanova AS, Tsyganov VE, Borisov AY, Tikhonovich IA, Staehelin C (2005) Nod factors induce nod factor cleaving enzymes in pea roots. Genetic and pharmacological approaches indicate different activation mechanisms. Plant Physiol 139:1051–1064
Owino WO, Ambuko JL, Mathooko FM (2005) New insights into cell wall disassembly during fruit ripening. Stewart Postharvest Rev 1:43–52
Papadopoulou K, Melton RE, Leggett M, Daniels MJ, Osbourn AE (1999) Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci USA 96:12923–12928
Parre E, Geitmann A (2005) More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol 137:274–286
Patterson SE (2001) Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol 126:494–500
Peng L, Kawagoe Y, Hogan P, Delmer D (2002) Sitosterol-beta-glucoside as primer for cellulose synthesis in plants. Science 295:147–150
Peterbauer T, Lahuta LB, Blochl A, Mucha J, Jones DA, Hedley CL, Gorecki RJ, Richter A (2001) Analysis of raffinose family oligosaccharide pathway in pea seeds with contrasting carbohydrate composition. Plant Physiol 127:1764–1772
Popper ZA, Fry SC (2003) Primary cell wall composition of bryophytes and charophytes. Ann Bot (Lond) 91:1–12
Queirolo CB, Andrea CS, Niemeyer HM, Couicuera LJ (1983) Inhibition of ATPase from chloroplasts by hydroxamic acid from the Gramineae. Phytochemistry 22:2455–2458
Rask L, Andreasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J (2000) Myrosinase: gene family evolution and herbivore defence in Brassicaceae. Plant Mol Biol 42:93–113
Rayon C, Lerouge P, Faye L (1998) The protein N-glycosylation in plants. J Exp Bot 326:1463–1472
Reiter WD (2002) Biosynthesis and properties of the plant cell wall. Curr Opin Plant Biol 5:536–542
Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Rodriguez-Llorente ID, Perez-Hormaeche J, El Mounadi K, Dary M, Caviedes MA, Cosson V, Kondorosi A, Ratet P, Palomares AJ (2004) From pollen tubes to infection threads: recruitment of Medicago floral pectic genes for symbiosis. Plant J 39:587–598
Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54:513–524
Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Rose JK, Braam J, Fry SC, Nishitani K (2002) The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 43:1421–1435
Sampedro J, Sieiro C, Revilla G, Gonzalez-Villa T, Zarra I (2001) Cloning and expression pattern of a gene encoding an alpha-xylosidase active against xyloglucan oligosaccharides from Arabidopsis. Plant Physiol 126:910–920
Sander L, Child R, Ulvskov P, Albrechtsen M, Borkhardt B (2001) Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape (Brassica napus) and Arabidopsis thaliana: evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth. Plant Mol Biol 46:469–479
Saint-Jore-Dupas C, Nebenfuhr A, Boulaflous A, Follet-Gueye ML, Plasson C, Hawes C, Driouich A, Faye L, Gomord V (2006) Plant N-glycan processing enzymes employ different targeting mechanisms for their spatial arrangement along the secretory pathway. Plant Cell 18:3182–3200
Scheible WR, Pauly M (2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr Opin Plant Biol 7:285–295
Schlumbaum A, Mauch F, Vogeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324:365–367
Schultze M, Kondorosi A (1998) Regulation of symbiotic root nodule development. Annu Rev Genet 32:33–57
Schultze M, Staehelin C, Brunner F, Genetet I, Legrand M, Fritig B, Kondorosi E, Kondorosi A (1998) Plant chitinase/lysozyme isoforms show distinct substrate specificity and cleavage site preference towards lipochitooligosaccharide Nod signals. Plant J 16:571–580
Schulz M, Weissenböck G (1987) Partial purification and characterization of a luteolin-triglucuronide-specific β-glucuronidase from rye primary leaves (Secale cereale). Phytochemistry 26:933–937
Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54:525–533
Showalter AM (1993) Structure and function of plant cell wall proteins. Plant Cell 5:9–23
Sicker D, Frey M, Schulz M, Gierl A (2000) Role of benzoxazinones in the survival strategy of plants. Int Rev Cytol 198:319–347
Simmons CR (1994) The physiology and molecular biology of plant 1,3-β-glucanases and 1,3;1,4-β-glucanases. Crit Rev Plant Sci 13:325–387
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Staehelin C, Schultze M, Kondorosi E, Kondorosi A (1995) Lipo-chitooligosaccharide nodulation signals from Rhizobium meliloti induce their rapid degradation by the host plant alfalfa. Plant Physiol 108:1607–1614
Staehelin C, Schultze M, Kondorosi E, Mellor RB, Boller T, Kondorosi A (1994) Structural modifications in Rhizobium meliloti Nod factors influence their stability against hydrolysis by root chitinases. Plant J 5:319–330
Stolle-Smits T, Beekhuizen JG, Kok MT, Pijnenburg M, Recourt K, Derksen J, Voragen AG (1999) Changes in cell wall polysaccharides of green bean pods during development. Plant Physiol 121:363–372
Steele NM, Sulova Z, Campbell P, Braam J, Farkas V, Fry SC (2001) Ten isoenzymes of xyloglucan endotransglycosylase from plant cell walls select and cleave the donor substrate stochastically. Biochem J 355:671–679
Strasser R, Schoberer J, Jin C, Glossl J, Mach L, Steinkellner H (2006) Molecular cloning and characterization of Arabidopsis thaliana Golgi alpha-mannosidase II, a key enzyme in the formation of complex N-glycans in plants. Plant J 45:789–803
Sturm A, Johnson KD, Szumilo T, Elbein AD, Chrispeels MJ (1987) Subcellular localization of glycosidases and glycosyltransferases involved in the processing of the N-linked oligosaccharides. Plant Physiol 85:741–745
Sue M, Ishihara A, Iwamura H (2000a) Purification and characterization of a beta-glucosidase from rye (Secale cereale L.) seedlings. Plant Sci 155:67–74
Sue M, Ishihara A, Iwamura H (2000b) Purification and characterization of a hydroxamic acid glucoside beta-glucosidase from wheat (Triticum aestivum L.) seedlings. Planta 210:432–438
Sue M, Yamazaki K, Yajima S, Nomura T, Matsukawa T, Iwamura H, Miyamoto T (2006) Molecular and structural characterization of hexameric beta-d-glucosidases in wheat and Rye. Plant Physiol 141:1237–1247
Sun Z, Duke SH, Henson CA (1995) The role of pea chloroplast alpha glucosidase in transitory starch degradation. Plant Physiol 108:211–217
Suzuki H, Takahashi S, Watanabe R, Fukushima Y, Fujita N, Noguchi A, Yokoyama R, Nishitani K, Nishino T, Nakayama T (2006) An isoflavone conjugate-hydrolyzing beta-glucosidase from the roots of soybean (Glycine max) seedlings. Purification, gene cloning, phylogenetics, and cellular localization. J Biol Chem 281:30251–30259
Szumilo T, Kaushal GP, Hori H, Elbein AD (1986) Purification and properties of a glycoprotein processing (-mannosidase from mung bean seedling. Plant Physiol 81:383–389
Taylor MA, Ross HA, McRae D, Stewart D, Roberts I, Duncan G, Wright F, Millam S, Davies HV (2000) A potato alpha-glucosidase gene encodes a glycoprotein-processing alpha-glucosidase II-like activity. Demonstration of enzyme activity and effects of down-regulation in transgenic plants. Plant J 24:305–316
Tevini M, Steinmuller D (1985) Composition and function of plastoglobuli II Lipid composition of leaves and plastoglobuli during beech leaf senescence. Planta 163:91–96
Thompson DS (2005) How do cell walls regulate plant growth? J Exp Bot 56:2275–2285
Tiné MAS, Cortelazzo AL, Buckeridge MS (2000) Xyloglucan mobilisation in cotyledons of developing plantlets of Hymenaea courbaril L. (Leguminosae-Caesalpinoideae). Plant Sci 154:117–126
Tymowska-Lalanne Z, Kreis M (1998) The plant invertases: physiology, biochemistry and molecular biology. Adv Bot Res 28:71–117
Umemoto N, Kakitani M, Iwamatsu A, Yoshikawa M, Yamaoka N, Ishida I (1997) The structure and function of a soybean beta-glucan-elicitor-binding protein. Proc Natl Acad Sci USA 94:1029–1034
Van Hengel AJ, Van Kammen A, De Vries SC (2002) A relationship between seed development, arabinogalactan-proteins (AGPs) and the AGP mediated promotion of somatic embryogenesis. Physiol Plant 114:637–644
Van der Holst PPG, Schlaman HRM, Spaink HP (2001) Proteins involved in the production and perception of oligosaccharides in relation to plant and animal development. Curr Opin Struct Biol 11:608–616
Van Damme EJ, Charels D, Roy S, Tierens K, Barre A, Martins JC, Rouge P, Van Leuven F, Does M, Peumans WJ (1999) A gene encoding a hevein-like protein from elderberry fruits is homologous to PR-4 and class V chitinase genes. Plant Physiol 119:1547–1556
Varki A, Cummings RD, Richard, Esko J, Freeze H, Hart G, Marth J (1999) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Boston
Verdoucq L, Moriniere J, Bevan DR, Esen A, Vasella A, Henrissat B, Czjzek M (2004) Structural determinants of substrate specificity in family 1 β-glucosidases: novel insights from the crystal structure of sorghum dhurrinase-1, a plant β-glucosidase with strict specificity, in complex with its natural substrate. J Biol Chem 279:31796–31803
Vierheilig H, Alt M, Neuhaus JM, Boller T, Wiemken A (1993) Colonization of transgenic Nicotiana sylvestris plants, expressing different forms of Nicotiana tabacum chitinase, by the root pathogen Rhizoctonia solani and by the mycorrhizal symbiont Glomus Mosseae. Mol Plant Microbe Interact 6:261–264
Vincken JP, Schols HA, Oomen RJ, McCann MC, Ulvskov P, Voragen AG, Visser RG (2003) If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol 132:1781–1789
Whetten R, MacKay JJ, Sederoff R (1998) Recent advances in understanding lignin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 49:585–609
Wingler A, Fritzius T, Wiemken A, Boller T, Aeschbacher R (2000) Trehalose induces the ADP-glucose pyrophosphorylase gene, APL3, and starch synthesis in Arabidopsis. Plant Physiol 124:105–114
Wingler A, Purdy S, MacLean JA, Pourtau N (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57:391–399
Wink M (1999) Introduction: biochemistry, role and biotechnology of secondary metabolism. In: Wink M (ed) Bochemistry of plant secondary metabolism. Annual plant reviews, vol 2. Sheffield Academic and CRC, Sheffield and Boca, pp 1–16
Woo KK, Miyazaki M, Hara S, Kimura M, Kimura Y (2004) Purification and characterization of a co(II)-sensitive alpha-mannosidase from Ginkgo biloba seeds. Biosci Biotechnol Biochem 68:2547–2556
Zhu Q, Maher EA, Masoud S, Dixon RA, Lamb CJ (1994) Enhanced protection against fungal attack by constitutive co-expression of chitinase and glucanase genes in transgenic tobacco. Bio/Technology 12:807–812
Zagrobelny M, Bak S, Rasmussen AV, Jørnensen B, Naumann CM, Møller BL (2004) Cyanogenic glucosides and plant-insect interactions. Phytochemistry 65:293–306
Xue J, Lenman M, Falk A, Rask L (1992) The glucosinolate-degrading enzyme myrosinase in Brassicaceae is encoded by a gene family. Plant Mol Biol 18:387–398
Yokoyama R, Nishitani K (2004) Genomic basis for cell-wall diversity in plants. A comparative approach to gene families in rice and Arabidopsis. Plant Cell Physiol 45:1111–1121
Zagrobelny M, Bak S, Rasmussen AV, Jorgensen B, Naumann CM, Moller BL (2004) Cyanogenic glucosides and plant-insect interactions. Phytochemistry 65:293–306
Zeng YC, Elbein AD (1998) Purification to homogeneity and properties of plant glucosidase I. Arch Biochem Biophys 355:26–34
Zhu J, Chen S, Alvarez S, Asirvatham VS, Schachtman DP, Wu Y, Sharp RE (2006) Cell wall proteome in the maize primary root elongation zone. I. Extraction and identification of water-soluble and lightly ionically bound proteins. Plant Physiol 140:311–325
Acknowledgments
I wish to thank Dr. Guy Hervé and Leanne Enns for the critical review of the manuscript. I thank two anonymous reviewers for their helpful comments and suggestions for improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minic, Z. Physiological roles of plant glycoside hydrolases. Planta 227, 723–740 (2008). https://doi.org/10.1007/s00425-007-0668-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0668-y