Abstract.
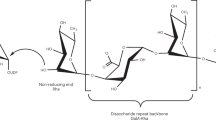
A solubilized α1,4-galacturonosyltransferase (GalAT) from tobacco transfers galacturonic acid (GalA) residues from UDP-GalA onto oligogalacturonide (OGA) exogenous acceptors with degrees of polymerization greater than nine (R.L. Doong and D. Mohnen 1998, Plant J 13: 363–374). The solubilized GalAT has been identified as putative polygalacturonate 4-α-galacturonosyltransferase (PGA-GalAT, EC 2.4.1.43) based on its α1,4-galacturonosyltransferase activity and similar K m for UDP-GalA, pH optimum and V max to those of membrane-bound PGA-GalAT (R.L. Doong et al., 1995, Plant Physiol 109: 141–152). The direction of elongation of homogalacturonan catalyzed by solubilized GalAT from microsomes of tobacco (Nicotiana tabacum L. cv. Samsun) cell suspensions has now been determined. Three different types of exogenous acceptor were used to study the direction of synthesis of homogalacturonan: unmodified OGAs, OGAs derivatized by biotinylation at the reducing end, and OGAs containing a 4,5-unsaturated GalA at the non-reducing end. The unmodified OGAs and the OGAs modified at the reducing end functioned equally well as acceptors in the galacturonosyltransferase reaction. In contrast, OGAs with the 4,5-unsaturated residue at the non-reducing end were not acceptors for homogalacturonan biosynthesis. These results show that homogalacturonan biosynthesis by solubilized GalAT occurs via the addition of GalA to the non-reducing end of the polymer chain.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 18 June 1998 / Accepted: 22 August 1998
Rights and permissions
About this article
Cite this article
Scheller, H., Doong, R., Ridley, B. et al. Pectin biosynthesis: a solubilized α1,4-galacturonosyltransferase from tobacco catalyzes the transfer of galacturonic acid from UDP-galacturonic acid onto the non-reducing end of homogalacturonan. Planta 207, 512–517 (1999). https://doi.org/10.1007/s004250050511
Issue Date:
DOI: https://doi.org/10.1007/s004250050511