Abstract
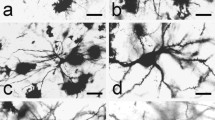
The morphology and distribution of parvalbumin-immunoreactive neurons (PV-ir) were studied in the human claustrum. PV-ir neurons were observed throughout the claustrum, with the highest numbers noted in the central (broadest) portion as compared with the dorsal and ventral aspects. Reaction product was evident in the neuronal perikarya, dendritic processes, and spines. In the majority of these labeled neurons, the cytoplasm was devoid of lipofuscin pigment. Cell bodies varied widely in both shape and size, ranging from oval and small, to multipolar and large. PV-ir neurons were classified into two groups, primarily based on dendritic morphology: spiny neurons with long and straight dendrites, and aspiny neurons with thin and curving dendritic processes. PV-ir fibers were seen throughout the neuropil, with many immuno-positive puncta noted.



















Similar content being viewed by others
References
Amadeo A, Ortino B, Frassoni C (2001) Parvalbumin and GABA in the developing somatosensory thalamus of the rat: an immunocytochemical ultrastructural correlation. Anat Embryol (Berl) 203:109–119
Andressen C, Blumke I, Celio MR (1993) Calcium-binding proteins: selective markers of nerve cells. Cell Tissue Res 271:181–208
Ashwell KWS, Hardman C, Paxinos G (2004) The claustrum is not missing from all monotreme brains. Brain Behav Evol 64:223–241
Baimbridge KG, Celio MR, Rogers JH (1992) Calcium-binding proteins in the nervous system. Trends Neurosci 15:303–308
Beal MF, Mazurek MF, Ellison DW, Swartz KJ, McGarvey U, Bird ED, Martin JB (1988) Somatostatin and neuropeptide Y concentrations in pathologically graded cases of Huntington’s disease. Ann Neurol 23:562–569
Behan M, Haberly LB (1999) Intrinsic and efferent connections of the endopiriform nucleus in the rat. J Comp Neurol 408:532–548
Berchtold MW, Celio MR, Heizmann CW (1985) Parvalbumin in human brain. J Neurochem 45:235–239
Berke JJ (1960) The claustrum, the external capsule and the extreme capsule of Macaca mulatta. Neurology 115:297–321
Berlucchi C (1927) Richerche di fine anatomia sul claustrum e sull’ insula dell gate [Microscopic anatomy of the claustrum and insula of the cat]. Riv Sperim Freniatria 51:125–157
Blumcke I, Hof PR, Morrison JH, Celio MR (1990) Distribution of parvalbumin immnunoreactivity in the visual cortex of old world monkeys and humans. J Comp Neurol 301:417–432
Blumcke I, Hof PR, Morrison JH, Celio MR (1991) Parvalbumin in the monkey striate cortex: a quantitative immunoelectron microscopy study. Brain Res 554:237–243
Braak H (1980) Architectonics of the human telencephalic cortex. In: Braitenberg V, Barlow HB, Florey E, Grusser OJ, van der Loos H (eds) Studies of brain function , vol 4. pp 1–147. Berlin-Heidelberg, Springer
Braak H (1983) Transparent Golgi impregnations: a way to examine both details of cellular processes and components of the nerve cell body. Stain Tecnol 58:91–95
Braak H, Braak E (1982) Neuronal types in the claustrum of man. Anat Embryol 163:473–488
Braak H, Braak E (1985) Golgi preparations as a tool in neuropathology with particular reference to investigations of the human telencephalic cortex. Progr Neurobiol 25:93–139
Braak H, Braak E, Ohm T, Bohl J (1988) Silver impregnation of Alzheimer neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol 63:197–200
Braak E, Schmidt S, Braak H, Celio M (1991) Parvalbumin-immunoreactive structures in the entorhinal cortex of the human adult. Soc Neurosci Abstr 17:972
Brand S (1981) A serial section Golgi analysis of the primate claustrum. Anat Embryol 162:447–460
Braun K, Scheich H, Schachner M, Heizmann CW (1985a) Distribution of parvalbumin, cytochrome oxidase activity and (14C)2-deoxyglucose uptake in the brain of the zebra finch. I. Auditory and vocal motor systems. Cell Tissue Res 240:101–115
Braun K, Scheich H, Schachner M, Heizmann CW (1985b) Distribution of parvalbumin, cytochrome oxidase activity and (14C)2-deoxyglucose uptake in the brain of the zebra finch II. Visual system. Cell Tissue Res 240:117–127
Braun K, Scheich H, Zuschratter W, Heizmann C, Matute C, Streit P (1988) Postnatal development of parvalbumin-, calbindin- and adult GABA-immunoreactivity in two visual nuclei of zebra finches. Brain Res 475:205–217
Brockhaus H (1940) Cytoarchitectural and myeloarchitectural study of claustral cortex and claustrum in man. J Psychol Neurol 49:249–348
Bruen PD, McGeown WJ, Shanks MF, Venneri A (2008) Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain 131:1355–2463
Burdach KF (1822) Von Baue und Leben des Gehirns (Leipzig 1822)
Cajal RS (1911) Histologie du Systeme Nerveux de l’Homme et des Vertebretes (Paris 1911)
Carey RG, Neal TL (1985) The rat claustrum; afferent and efferent connections with visual cortex. Brain Res 329:185–193
Carey RG, Bear MF, Diamond IT (1980) The laminar organization of the reciprocal projections between the claustrum and the striate cortex in the tree shrew, Tupaia glis. Brain Res 184:193–198
Cascella NG, Gerner GJ, Fieldstone SC, Sawa A, Schretlen DJ (2011) The insula-claustrum region and delusions in schizophrenia. Schizophr Res 133:77–81
Celio MR (1984) Parvalbumin is a marker of fast-firing neurons. Neurosci Lett [Suppl.] 18:332
Celio MR (1986) Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science 231:995–997
Celio MR (1990) Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35:375–475
Celio MR, Heizmann CW (1981) Calcium-binding protein parvalbumin is a neuronal marker. Nature 293:300–302
Celio MR, Baier W, Schärer L, de Viragh PA, Gerday C (1988) Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium 9(2):81–86
Cicchetti F, Parent A (1996) Striatal interneurons in Huntington’s disease: selective increase in the density of calretinin-immunoreactive medium-sized neurons. Mov Disord 11:619–626
Contestabile A (2000) Role of NMDA receptor activity and nitric oxide production in brain development. Brain Res Rev 32:476–509
Cowan RL, Wilson CJ, Emson PC (1990) Parvalbumin is present in GABA-containing interneurons in the rat neostriatum. J Comp Neurol 302:198–205
Crick FC, Koch C (2005) What is the function of the claustrum? Philos Trans Roy Soc B 1271–1279
Czeiger D, White EL (1997) Comparison of the distribution of parvalbumin-immunoreactive and other synapses onto the somata of callosal projection neurons in mouse visual and somatosensory cortex. J Comp Neurol 379:198–210
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D (28K) immunohistochemistry. Brain 122:1421–1436
Davis WB (2008) The claustrum in autism and typically developing male children: a quantitative MRI study. Brigham Young University, Thesis
De Biasi S, Arcelli P, Spreafico R (1994) Parvalbumin immunoreactivity in the thalamus of guinea pig: light- and electron-microscopic correlation with gamma-amino-butyric acid immunoreactivity. J Comp Neurol 348:556–569
De Felipe J (1997) Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 14:1–19
De Vries E (1910) Bemerkungen zur ontogenie und vergleichen-den anatomie des ckaustrums. Folia Neurobiol 4:481–513
Del Rio MR, De Felipe J (1997) Colocalization of parvalbumin and calbindin D-28k in neurons including chandelier cells of the human temporal neocortex. J Chem Neuroanat 12:165–173
Demeulemeester H, Vandesande F, Orban GA, Heizmann CW, Pochet R (1989) Calbindin D-28K and parvalbumin immunoreactivity is confined to two separate neuronal subpopulations in the cat visual cortex, whereas partial coexistence is shown in the dorsal lateral geniculate nucleus. Neurosci Lett 99:6–11
Dinopoulos A, Papadopoulos GC, Michaloudi H, Parnavelas JG, Uylings HS, Karamanlidis AN (1992) Claustrum in the hedgehog Erinaceus europaeus brain: cytoarchitecture and connections with cortical and subcortical structures. J Comp Neurol 316:187–205
Druga R (1966a) The claustrum of the cat (Felis domestica). Folia Morphol (Praha) 14:7–16
Druga R (1966b) Cortico-claustral connections. I. Fronto-claustral connections. Folia Morphol (Praha) 14:391–399
Druga R (1968) Cortico-claustral connections. II. Connections from the parietal, temporal and occipital cortex to the claustrum. Folia Morphol (Praha) 16:142–149
Druga R (1971) Projection of prepyriform cortex into claustrum. Folia Morphol (Praha) 19:405–410
Druga R (1974) The claustrum and the transitional neopaleo-cortical area of the hedgehog (Erinacea europaeus). Anat Anz (Jena) 135:442–454
Druga R (1975) Claustrum (Struktura, Ontogenese a Spoje), Doctoral dissertation, Charles University, Praha, p 193
Druga R, Chen S, Bentivoglio M (1993) Parvalbumin and calbindin in the rat claustrum; an immunocytochemical study combined with retrograde tracing from frontoparietal cortex. J Chem Neuroanat 6:399–406
Edelstein LR (1986) The anatomy of the claustrum: a light- and electron-microscopic analysis in rat and monkey incorporating the technique of HRP cytochemistry, Doctoral dissertation, State University of New York at Stony Brook, New York, p 279. (incl. 66 figures)
Edelstein LR, Denaro FJ (1979) The monkey claustrum: an electron-microscopic analysis. Soc Neurosci Abstr 5:428 (#1444)
Edelstein LR, Denaro FJ (1980) The rat claustrum: a light- and electron-microscopic analysis. Soc Neurosci Abstr 6:735 (#247.25)
Edelstein LR, Denaro FJ (2004a) The neurobiology of consciousness and Sir Francis Crick. Cell Mol Biol 50:671–673
Edelstein LR, Denaro FJ (2004b) The claustrum: a historical review of its anatomy, physiology, cytochemistry and functional significance. Cell Mol Biol 50:675–702
Edelstein LR, Cozzi B, Castagna M, Quilici F, Lenzi C, Piano I, Pirone A (2010) Parvalbumin and neuropeptide Y immunoreactivity in the human claustrum. Society for Neuroscience, 40th Annual Meeting, Abstract #900.1
Edelstein L, Denaro F, Stamm J, Landzhov B, Malinova L, Hinova-Palova D, Paloff A, Bozhilova-Pastirova A, Dzhambazova E, Bocheva A, Ovtscharoff W (2011a) Distribution of CB1 receptors in the claustrum of rats undergoing acute stress: an immunohistochemical study. Society for Neuroscience, 41st Annual Meeting, #734.12
Edelstein L, Hinova-Palova D, Malinova L, Papantchev V, Landzhov B, Paloff A, Ovtscharoff W (2011b) Distribution of neuropeptide Y immunoreactivity in the dorsal claustrum of the cat: light- and electron-microscopic identification of distinct neuronal populations. Society for Neuroscience, 41st Annual Meeting, #817.19
Edelstein L, Hinova-Palova D, Denaro F, Landzhov B, Malinova L, Minkov M, Paloff A, Ovtscharoff W (2012a) “NADPH-diaphorase-positive neurons in the human claustrum,” Society for Neuroscience, 42nd Annual Meeting, #895.20
Edelstein L, Hinova-Palova D, Landzhov B, Malinova L, Minkov M, Paloff A, Ovtscharoff W (2012b) Neuronal nitric oxide synthase immunoreactivity in the human claustrum: light- and electron microscopic investigation. Society for Neuroscience, 42nd Annual Meeting, #895.21
Filimonoff IN (1966) The claustrum: its origin and development. J Hirnforsch 8:503–528
Gabbott PL, Bacon SJ (1995) Co-localization of NADPH diaphorase activity and GABA immunoreactivity in local circuit neurons in the medial prefrontal cortex (mPFC) of the rat. Brain Res 699:321–328
Gartner U, Hartig W, Brauer K, Bruckner G, Arendt T (2001) Electron-microscopic evidence for different myelination of rat septohippocampal fibers. NeuroReport 12:17–20
Gaykema R, Zaborszky L (1997) Parvalbumin-containing neurons in the basal forebrain receive direct input from the substantia nigra-ventral tegmental area. Brain Res 747:173–179
German DC, Manaye KF, Sonsalla PK, Brooks BA (1992) Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin D28k-containing cells. Ann NY Acad Sci 648:42–46
Glezer II, Hof PR, Morgane PJ (1998) Comparative analysis of calcium-binding protein-immunoreactive neuronal populations in the auditory and visual systems of the bottlenose dolphin (Tursiops truncatus) and the macaque monkey (Macaca fascicularis). J Chem Neuroanat 15:203–237
Gomez-Urquijo SM, Guitierrez-Ibarluzae I, Bueno-Lopez JL, Roblet C (2000) Percentage incidence of gamma-aminobutyric acid neurons in the claustrum of the rabbit and comparison with the cortex and putamen. Neurosci Lett 282:177–180
Guirado S, Real MA, Olmos JL, Davila JC (2003) Distinct types of nitric oxide-producing neurons in the developing and adult mouse claustrum. J Comp Neurol 465:431–444
Heizmann CW (1984) Parvalbumin, an intercellular calcium-binding protein: distribution, properties and possible roles in mammalian cells. Experientia 40:910–921
Heizmann CW, Celio MR (1987) Immunolocalization of parvalbumin. Meth Enzymol 139:552–570
Heizmann CW, Hunziker W (1991) Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci 16:98–103
Hendry SHC, Jones EG, Emson PC, Lawson DEM (1989) Two classes of cortical GABA neurons defined by differential calcium binding proteins immunoreactivity. Exp Brain Res 76:467–472
Hershberger ME, Bruns ME, Bruns ME, Song Y, German DC (2008) Cabp 1—calcium binding protein 1. Nature Genetics
Hinova-Palova D (1981) Identification of degenerated boutons in claustrum dorsale after lesion of visual cortex. CR Acad Bulg Sci 34:449–452
Hinova-Palova D (1986) Light-microscopic and ultrastructural organization of the claustrum in the cat. Afferent and efferent connections. Dissertation, vol I.-text, p 211, vol II.-256 text-figures, Medical Academy, Sofia
Hinova-Palova DV, Braak E (1994) Parvalbumin immunoreactive neurons in human claustrum. National conference of Anatomy, Histology and Embryology (Stara Zagora)
Hinova-Palova DV, Paloff AM (1982) Corticoclaustral connections. An electron-microscopic study. Verh Anat Ges 76:503–504
Hinova-Palova D, Paloff A (1984) Identification of degenerated synaptic boutons in the claustrum of cat after lesion of the parietal cortex. Contemp Probl Neuromorphol (Sofia) 13–14:154–160
Hinova-Palova DV, Paloff AM, Usunoff KG (1980a) Identification of three types of degenerated boutons in claustrum dorsale of the cat after lesion of the temporal cortex. CR Acad Bulg Sci 33:125–128
Hinova-Palova DV, Paloff AM, Usunoff KG (1980b) Identification of three types of degenerated boutons in claustrum dorsale of the cat after lesion of the frontal cortex. CR Acad Bulg Sci 33:129–132
Hinova-Palova DV, Dimova R, Ivanov DP (1987) Identification of small neurons (dwarf cells) in the claustrum of the cat. Light and electron microscopic observations. Verh Anat Ges Leipzig, p 66
Hinova-Palova DV, Paloff AM, Usunoff KG (1987) Dendrodendritic and axoaxonal synapses, and synaptoid contacts in the dorsal claustrum of the cat III. In: Symposium on Peripheral and Central Synapses, Varna, Programme and Abstracts
Hinova-Palova DV, Paloff AM, Usunoff KG, Dimova RN, Yossifov TY, Ivanov DP (1988) Reciprocal connections between the claustrum and the auditory cortcal fields in the cat. An experimental study using light- and electron microscopic anterograde degeneration methods, and horseradish peroxidase retrograde axonal transport. J Hirnforsch 29:255–278
Hinova-Palova DV, Paloff AM, Usunoff KG (1991) Identification of eight neuronal types in the cat’s claustrum. A combined Golgi and electron microscope study. Verh Anat Ges 84(Anat Anz Suppl 168):685–687
Hinova-Palova DV, Paloff AM, Christova T, Ovtscharoff WA (1997) Topographical distribution of NADPH-diaphorase-positive neurons in the cat’s claustrum. Eur J Morphol 35:105–116
Hinova-Palova DV, Paloff AM, Bozhilova-Pastirova AI, Ovtscharoff W (1998) Ultrastructural localization of GABA and parvalbumin (PV) in the cat claustrum. Verh Anat Ges 93:117
Hinova-Palova DV, Christova T, Yotovski PV, Logofetov AP, Paloff AM (2001) Somatostatin-like neurons and fibres in the cat claustrum. CR Acad Bulg Sci 54:81–84
Hinova-Palova DV, Edelstein LR, Paloff AM, Hristov S, Papantchev VG, Ovtscharoff WA (2007) Parvalbumin in the cat claustrum: ultrastructure, distribution and functional implications. Acta Histochem 109:61–77
Hinova-Palova DV, Edelstein LR, Paloff AM, Hristov S, Papantchev VG, Ovtscharoff WA (2008) Neuronal nitric oxide synthase immunopositive neurons in cat claustrum; a light and electron microscopic study. J Mol Histol 39(4):447–457
Hinova-Palova D, Edelstein L, Papantchev V, Landzhov B, Malinova L, Todorova-Papantcheva D, Minkov M, Paloff A, Ovtscharoff A (2012) Light- and electron-microscopic study of leucine enkephalin immunoreactivity in the cat claustrum. J Mol Histol 43(6):641–649
Höchli M, Zetzsche T, Chan-Palay V (1991) Parvalbumin-immunoreactive neurons in the normal human hippocampus. Dement Geriatr Cogn Disord 2:243–258
Hof PR, Nimchinsky EA, Celio MR, Bouras C, Morrison JH (1993) Calretinin-immunoreactive neocortical interneurons are unaffected in Alzheimer’s disease. Neurosci Lett 152:145–148
Hontanilla B, Parent A, Gimenez-Amaya JM (1995) Heterogeneous distribution of neurons containing calbindin D-28k and/or parvalbumin in the rat red nucleus. Brain Res 696:121–126
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Iacopino A, Christakos S, German DC, Sonsalla PK, Altar CA (1992) Calbindin-D28k-containing neurons in animal models of neurodegeneration: possible protection from excitotoxicity. Mol Brain Res 13:251–261
Idrizbegovic E, Bogdanovic N, Canlon B (1999) Sound stimulation increases calcium-binding protein immunoreactivity in the inferior colliculus in mice. Neurosci Lett 259:49–52
Idrizbegovic E, Canlon B, Bross LS, Willott JF, Bogdanovic N (2001) The total number of neurons and calcium-binding protein-positive neurons during aging in the cochlear nucleus of CBA/CaJ mice: a quantitative study. Hear Res 158:102–115
Idrizbegovic E, Bogdanovic N, Viberg A, Canlon B (2003) Auditory peripheral influences on calcium-binding protein immunoreactivity in the cochlear nucleus during aging in the C57BL/6J mouse. Hear Res 179:33–42
Kagi U, Berchtold MW, Heizmann CW (1987) Ca2+-binding parvalbumin in rat testis. Characterization, localization and expression during development. J Biol Chem 262:7314–7320
Kalaitzakis MR, Pearce RK, Gentleman SM (2009) Clinical correlates of pathology in the claustrum in Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 461:12–15
Kemp JM, Powell TPS (1970) The corticostriate projection in the monkey. Brain 93:525–546
Kevetter GA (1996) Pattern of selected calcium-binding proteins in the vestibular nuclear complex of two rodent species. J Comp Neurol 365:575–584
Khachaturian H, Lewis M, Hollt V, Watson S (1983) Telencephalic enkephalinergic system in the rat brain. J Neurosci 3:844–855
Kita H, Kosaka T, Heizmann CW (1990) Parvalbumin-immuno-reactive neurons in the rat neostriatum: a light- and electron-microscopic study. Brain Res 536:1–15
Kosaka T, Heizmann CW (1989) Selective staining of a population of parvalbumin-containing GABAergic neurons in the rat cerebral cortex by lectins with specific affinity for terminal N-acetylgalactosamine. Brain Res 483:158–163
Kowall NW, Beal MF (1988) Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: normal anatomy and alterations in Alzheimer’s disease. Ann Neurol 23:105–114
Kowianski P, Morys J, Karwacki Z, Dziewiatkowski J, Narkiewicz O (1998) The cortico-related zones of the rabbit claustrum: study of the claustrocortical connections based on the retrograde axonal transport of fluorescent tracers. Brain Res 784:199–209
Kowianski P, Morys JM, Wojcik S, Dziewiatkowski J, Morys J (2003) Co-localization of NOS with calcium-binding proteins during the postnatal development of the rat claustrum. Folia Morphol 62:211–214
Kowianski P, Morys JM, Wojcik S, Dziewiatkowski J, Luczynska A, Spodnik E et al (2004) Neuropeptide-containing neurons in the endopiriform region of the rat: morphology and colocalization with calcium-binding proteins and nitric oxide synthase. Brain Res 996:97–110
Kowianski P, Dziewiatkowski J, Morys JM, Majak K, Wojcik S, Edelstein LR, Lietzau G, Morys J (2009) “Colocalization of neuropeptides with calcium-binding proteins in the claustral interneurons during postnatal development of the rat.” Brain Research Bulletin, 80/3:100–106
Kunzle H (1975) Bilateral projection from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88:195–209
Kunzle H (1978) An autoradiographic analysis of the efferent connections from the premotor and adjacent prefrontal regions (area 6 and 9) in Macaca fascicularis. Brain Behav Evol 15:185–234
Landau E (1923) Zur kenntnis der Beziehungen des claustrums zum nucleus amygdalae und zur area piriformis im speziellen zum tractus olfactorius. Schweiz Arch Neurol Psychiatrie 13:391–400
Lavoie B, Parent A (1991) Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. NeuroReport 2:601–604
LeVay S (1986) Synaptic organization of claustrum and geniculate afferents to the visual cortex of the cat. J Neurosci 6:3564–3575
LeVay S, Sherk H (1981a) The visual claustrum of the cat. I. Structure and connections. J Neurosci 1:956–980
LeVay S, Sherk H (1981b) The visual claustrum of the cat. II. The visual field map. J Neurosci 1:981–992
Lewis DA, Curley AA, Glausier JR, Volk DW (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35:57–67
Lohmann C, Friauf E (1996) Distribution of the calcium-binding proteins parvalbumin and calretinin in the auditory brainstem of adult and developing rats. J Comp Neurol 367:90–109
Loo TT (1931) The forebrain of the opossum. Didelphis virginiana. J Comp Neurol 52:1–148
Macarico da Costa N, Fursinger D, Martin KAC (2010) The synaptic organization of the claustral projection to the cat’s visual cortex. J Neurosci 3122–10
Macchi G (1948) Morphology and structure of human claustrum. Cervello 24:1–26
Macchi G, Bentivoglio M, Minciacchi D, Molinari M (1983) Claustroneocortical projections studied in the cat by means of multiple retrograde fluorescent tracing. J Comp Neurol 215:121–134
Mamos L (1984) Morphology of claustral neurons in the rat. Folia Morphol (Warszawa) 43:73–78
Mathur BN, Caprioli RM, Deutch AY (2009) Proteomic analysis illuminates a novel structural definition of the claustrum and insula. Cereb Cortex 19(10):2372–2379
McDonald A, Betette RL (2001) Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and colocalization of calbindin-D28k. Neuroscience 102:413–425
Morys J, Berdel B, Maciejewska B, Sadowski M, Sidorowicz M, Kowianska J et al (1996) Division of the human claustrum according to its architectonics, morphometric parameters and cortical connections. Folia Morphol (Warszawa) 55:69–82
Mouatt-Prigent A, Agid Y, Hirsch EC (1994) Does the calcium binding protein calretinin protect dopaminergic neurons against degeneration in Parkinson’s disease? Brain Res 668:62–70
Münkle MC, Waldvogel HJ, Faull RL (2000) The distribution of calbindin, calretinin and parvalbumin immunoreactivity in the human thalamus. J Chem Neuroanat 19(3):155–173
Namavar MR, Sadeghi Y, Haghir H (2005) A new division of the human claustrum basis on the anatomical landmarks and morphological findings. J Iranian Anat Sci Spring 3(1):57–66
Narkiewicz O (1964) Degeneration in the claustrum after regional neocortical ablations in the cat. J Comp Neurol 123:335–336
Narkiewicz O (1972) Frontoclaustral interrelations in cats and dogs. Acta Neurobiol Exp (Warszawa) 32:141–150
Neal JW, Pearson RCA, Powell TPS (1986) The relationship between the auditory cortex and the claustrum in the cat. Brain Res 366:145–151
Norita M (1977) Demonstration of bilateral claustrocortical connections in the cat with the method of retrograde axonal transport of horseradish peroxidase. Arch Histol Japon 40:1–10
Olsen CR, Graybiel AM (1980) Sensory maps in the claustrum of the cat. Nature 288:479–481
Otellin VA, Makarov FN (1972) Descending connections of the auditory cortex of the cat with contralateral neostriatal complex and claustrum. Dokl Akad Nauk SSSR (Ser Biol) 202:723–725
Paloff AM (2000) Cytoarchitecture, ultrastructure, transmitter immunocytochemistry and connections of the inferior colliculus. D Sc dissertation, vol 1 and 2, Medical University, Sofia
Paloff AM, Hinova-Palova D (1984) Efferent projections of the temporal cortex. V. Topographic distribution of the projection fibers originating in the auditory field Ep and ending in the basal ganglia, thalamus and brain stem. Contemp Probl Neuromorphol (Sofia) 13–14:147–53
Paloff AM, Hinova-Palova DV (1998) Topographical distribution of NADPH-diaphorase-positive neurons in the cat’s inferior colliculus. J Hirnforsch 39:231–243
Paloff AM, Usunoff KG (1980) Identification of two types of degenerated boutons in colliculus inferior after lesion of the auditory cortex in cats. CR Acad Bulg Sci 33:125–128
Paloff AM, Hinova-Palova DV, Yotovski PV, Ovtscharoff WA (2001a) Coexistence of GABA and parvalbumin (PV) in cat inferior colliculus. In: XV National Congress of the Bulgarian Anatomical Society with international participation. Stara Zagora, 1–3 June Abstract, p 8
Paloff AM, Hinova-Palova DV, Yotovski PV, Ovtscharoff WA (2001b) Distribution of parvalbumin immunoreactivity in cat’s inferior colliculus: light- and electron-microscopical studies. VI. Edirne: National Congress of Anatomy, 3–7 September Abstract, p 93
Paloff A, Usunoff K, Yotovski P, Hinova-Palova D, Ovtscharoff W (2004) Parvalbumin-like immunostaining in the cat inferior colliculus. Light- and electron-microscopic investigation. Acta Histochem 106:219–234
Papantchev V, Paloff A, Christova T, Hinova-Palova D, Ovtscharoff W (2005) Light microscopical study of nitric oxide synthase I-positive neurons, including fibres in the vestibular nuclear complex of the cat. Acta Histochem 107:113–120
Parvizi J, Damasio AR (2003) Differential distribution of calbindin-D28k and parvalbumin among functionally distinctive sets of structures in the macaque brainstem. J Comp Neurol 462:153–167
Paxinos G, Watson C (1989) The rat brain in stereotaxic coordinates. Academic Press, New York
Pearson RCA, Brodal P, Gatter KC, Powell TPS (1982) The organization of the connections between the cortex and the claustrum in the monkey. Brain Res 234:435–441
Pilleri G (1961) The claustrum of Didelphis marsupialis Lin (Marsupialis, Didelphoidea). Acta Anat 45:310–314
Pilleri G (1962) The claustrum of the Canadian beaver (Castor canadensis Kuhl): structure and comparative anatomy. J Hirnforsch 5:59–81
Pirone A, Cozzi B, Edelstein L, Peruffo A, Lenzi C, Quilici F, Antonini R, Castagna M (2012) Topography of Gng2- and NetrinG2-expression suggests an insular origin of the human claustrum. PLoS One 7(9):e44745. doi:10.1371/journal.pone.0044745
Rae ASL (1954) The connections of the claustrum. Confin Neurol (Basel) 14:211–219
Rahman FE, Baizer JS (2007) Neurochemically defined cell types in the claustrum of the cat. Brain Research XX
Rajakumar N, Elisevich K, Flumerfelt BA (1994) Parvalbumin-containing GABAergic neurons in the basal ganglia output system of the rat. J Comp Neurol 350:324–336
Real MA, Davila JC, Guirado S (2003) Expression of calcium-binding proteins in the mouse claustrum. J Chem Neuroanat 25:151–160
Reynhout K, Baizer JS (1999) Immunoreactivity for calcium-binding proteins in the claustrum of the monkey. Anat Embryol 199:75–83
Ribak CE, Nitsh R, Seress L (1990) Proportion of parvalbumin-positive basket cells in the GABAergic interneurons of pyramidal and granule cells of the rat hippocampal formation. J Comp Neurol 300:449–461
Ribak CE, Seress L, Leranth C (1993) Electron-microscopic immunocytochemical study of the distribution of parvalbumin-containing neurons and axon terminals in the primate dentate gyrus and Ammon’s horn. J Comp Neurol 327:298321
Riche D, Lanoir J (1978) Some claustrocortical connections in the cat and baboon as studied by retrograde HRP transport. J Comp Neurol 177:435–444
Rogers JH (1987) Calretinin: a gene for novel calcium-binding protein expressed principally in neurons. J Cell Biol 105:1343–1353
Rogers JH (1992) Immunohistochemical markers in rat cortex: co-localization of calretinin and calbindin-D28k with neuropeptides and GABA. Brain Res 587:147–157
Rogers JH, Resibois A (1992) Calretinin and calbindin-D28k in rat brain: patterns of partial co-localization. Neuroscience 51:843–865
Rowniak M, Szteyn S, Robak A, Klawon M (1994) The types of neurons in the claustrum of Bison bonasus: Nissl and Golgi study. Folia Morphol 53:231–237
Sadowski M, Morys J, Jakubowska-Sadowska K, Narkiewicz O (1997) Rat’s claustrum shows two main cortico-related zones. Brain Res 756(147):52
Sampson VL, Morrison JH, Vickers JC (1997) The cellular basis for the relative resistance of parvalbumin and calretinin-immunoreactive neocortical neurons to the pathology of Alzheimer’s disease. Exp Neurol 145:295–302
Satoh J, Tabira T, Sano M, Nakayama H, Tateishi J (1991) Parvalbumin-immunoreactive neurons in the human central nervous system are decreased in Alzheimer’s disease. Acta Neuropathol 81:388–395
Schmidt S, Braak E, Braak H (1993) Parvalbumin-immunoreactive structures of the adult human entorhinal and transentorhinal region. Hippocampus 3(4):459–470
Schwaller B, Meyer M, Schiffmann S (2002) “New” functions for “old” proteins: the role of the calcium-binding proteins calbindin-D28k, calretinin and parvalbumin, in cerebellar physiology. Study with knockout mice. Cerebellum 1:241–258
Seress L, Nitsch R, Leranth C (1993) Calretinin immunoreactivity in the monkey hippocampal formation-I. Light- and-electron microscopic characteristics and co-localization with other calcium-binding proteins. Neuroscience 55:775–796
Sherk H (1986) The claustrum and the cerebral cortex. In: Jones EG, Peters A (eds) Cerebral cortex, vol 5. Plenum Press, New York, pp 467–499
Sloniewski P, Usunoff KG, Pilgrim C (1986a) Diencephalic and mesencephalic afferents of the rat claustrum. Anat Embryol (Berlin) 173:401–411
Sloniewski P, Usunoff KG, Pilgrim C (1986b) Retrograde transport of fluorescent tracers reveals extensive ipsi- and contralateral claustrocortical connections in the rat. J Comp Neurol 246:467–477
Smaluhn N, Plaschke M, Leranth C, Nitsch R (2000) The transentorhinal cortex of the African green monkey: a combined light- and electron-microscopic study of calcium-binding protein-containing neurons. Anat Embryol (Berlin) 202:143–158
Smith Y, Pare JF, Pare D (1998) Cat intra-amygdaloid inhibitory network: ultrastructural organization of parvalbumin-immunoreactive elements. J Comp Neurol 391:164–179
Smith Y, Pare JF, Pare D (2000) Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol 416:496–508
Smythies J, Edelstein L, Ramachandran VS (2012) Hypotheses relating to the function of the claustrum. Front Integr Neurosci 6:53. doi:10.3389/fnint.2012.00053
Soares-Mota M, Henze I, Mendez-Otero R (2001) Nitric oxide synthase-positive neurons in the rat superior colliculus: Co-localization of NOS with NMDAR1 glutamate receptor. GABA and parvalbumin. J Neurosci Res 64:501–507
Somogyi P, Takagi T (1982) A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience 4:1779–1784
Sorvari H, Soininen H, Paljärvi L, Karkola K, Pitkänen A (1995) Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol 360(2):185–212
Spahn B, Braak H (1985) Percentage of projection neurons and various types of interneurons in the human claustrum. Acta Anat (Basel) 122:245–248
Stelmasiak M (1955) Volume of the claustrum in man. Folia Morphol (Warszawa) 6:137–144
Stichel CC, Kägi U, Heizmann CW (1986) Parvalbumin in cat brain: isolation, characterization, and localization. J Neurochem 47(1):46–53
Stichel CC, Singer W, Heizmann CW, Norman AW (1987) Immunohistochemical localization of calcium-binding proteins, parvalbumin and calretinin-D 28k in the adult and developing visual cortex of cats: a light- and electron-microscopic study. J Comp Neurol 262:563–577
Stichel CC, Singer W, Heizmann CW (1988) Light- and electron- microscopic immunocytochemical localization of parvalbumin in the dorsal lateral geniculate nucleus of the cat: evidence for coexistence with GABA. J Comp Neurol 268:29–37
Stöcker K, Stöcker W, Ritter-Frank Y, Scriba PC (1985) Chemically activated glass slides for frozen sections and their use in autoantibody diagnosis (Article in German). Acta Histochem Suppl 31:283–294
Tanne-Gariepy J, Boussaoud D, Rouiller EM (2002) Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J Comp Neurol 454:140–157
Toth K, Freund TF (1992) Calbindin-D28k-containing non-pyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience pp 793–805
Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411
Ulfig N (2001) Expression of calbindin and calretinin in the human ganglionic eminence. Pediatr Neurol 24:357–360
Ulfig N, Chan WY (2001) Differential expression of calcium-binding proteins in the red nucleus of the developing and adult human brain. Anat Embryol (Berlin) 203:95–108
Valtschanoff JG, Weiberg RJ, Kharazia VN, Schmidt HH, Nakane M, Rustioni A (1993) Neurons in cerebral cortex that synthesize nitric oxide: NADPH diaphorase histochemistry, NOS immunocytochemistry and colocalization with GABA. Neurosci Lett 157:157–161
Vater M, Braun K (1994) Parvalbumin, calbindin-D28k, and calretinin immunoreactivity in the ascending auditory pathway of horseshoe bats. J Comp Neurol 341:534–558
Vecellio M, Schwaller B, Meyer M, Hunziker W, Celio MR (2000) Alteration in Purkinje cell spines of calbindin-D28k and parvalbumin knock-out mice. Eur J Neurosci 12:945–954
Vicq d’Azyr F (1786) Traité d’Anatomie et de Physiologie avec des Planches Coloriées Représentant au Naturel les Divers Organes de l’Homme et des Animaux, vol II, plates X, XI, XXII and XXVI (Paris, François Didot l’aîné, 1786)
Witter MP, Room P, Groenewegen HJ, Lohman AH (1988) Reciprocal connections of the insular and piriform claustrum with limbic cortex: an anatomical study in the cat. Neuroscience 24:519–539
Wojcik S, Dziewiatkowski J, Spodnik E, Ludkiewicz B, Domaradzka-Pytel B, Kowianski P et al (2004) Analysis of calcium-binding protein immunoreactivity in the claustrum and the endopiriform nucleus of the rabbit. Acta Neurobiol Exp 64:449–460
Yan XX, Jen LS, Garey LJ (1996) NADPH-diaphorase-positive neurons in primate cerebral cortex colocalize with GABA and calcium-binding proteins. Cereb Cortex 6:524–529
Zilles K, Zilles B, Schleicher A (1980) A quantitative approach to cytoarchitectonics. VI. The areal pattern of the cortex of the albino rat. Anat Embryol (Berlin) 159:335–360
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Braak: deceased.
Rights and permissions
About this article
Cite this article
Hinova-Palova, D.V., Edelstein, L., Landzhov, B.V. et al. Parvalbumin-immunoreactive neurons in the human claustrum. Brain Struct Funct 219, 1813–1830 (2014). https://doi.org/10.1007/s00429-013-0603-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0603-x