Abstract
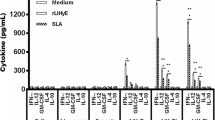
Visceral leishmaniasis (VL) is a tropical and subtropical disease which is endemic in more than eighty countries around the world. Leishmania infantum is one of the main causative agents of VL disease. Currently, there is no approved-to-market vaccine for VL therapy. In this study, we evaluated cellular and humoral immune responses induced by our newly designed multi-epitope vaccine in BALB/c mice. Four antigenic proteins, including histone H1, sterol 24-c-methyltransferase (SMT), Leishmania-specific hypothetical protein (LiHy), and Leishmania-specific antigenic protein (LSAP) were chosen for the prediction of potential immunodominant epitopes. Moreover, to enhance vaccine immunogenicity, two toll-like receptors 4 (TLR4) agonists, resuscitation-promoting factors of Mycobacterium tuberculosis (RpfE and RpfB), were employed as the built-in adjuvants. Immunization with the designed multi-epitope vaccine elicited a robust Th1-type immune response, compared to other groups, as shown by increased levels of IL-2, IFN-γ, TNF-α, and IgG2a. Furthermore, a significant decrease was observed in Th-2-type-related cytokines such as IL-4 in immunized mice. The designed construct also induced a significant reduction in parasite load (p < 0.0001), conferring protection against L. infantum challenge. This study could be promising in gaining insight towards the potential of peptide epitope-based vaccines as effective protective approaches against Leishmania species.





Similar content being viewed by others
References
Steverding D (2017) The history of leishmaniasis. Parasite Vector 10(1):82. https://doi.org/10.1186/s13071-017-2028-5
Rogers ME (2012) The role of leishmania proteophosphoglycans in sand fly transmission and infection of the Mammalian host. Front Microbiol 3:223–223. https://doi.org/10.3389/fmicb.2012.00223
Bates PA (2007) Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol 37(10–3):1097–1106. https://doi.org/10.1016/j.ijpara.2007.04.003
Kuhls K, Alam MZ, Cupolillo E, Ferreira GEM, Mauricio IL, Oddone R, Feliciangeli MD, Wirth T, Miles MA, Schönian G (2011) Comparative microsatellite typing of new world Leishmania infantum reveals low heterogeneity among populations and its recent old world origin. PLoS Negl Trop Dis 5(6):e1155. https://doi.org/10.1371/journal.pntd.0001155
Ready PD (2014) Epidemiology of visceral leishmaniasis. J Clin Epidemiol 6:147–154. https://doi.org/10.2147/CLEP.S44267
Nagle AS, Khare S, Kumar AB, Supek F, Buchynskyy A, Mathison CJN, Chennamaneni NK, Pendem N, Buckner FS, Gelb MH, Molteni V (2014) recent developments in drug discovery for Leishmaniasis and human African Trypanosomiasis. Chem Rev 114(22):11305–11347. https://doi.org/10.1021/cr500365f
Oryan A (2015) Plant-derived compounds in treatment of leishmaniasis. IJVR 16(1):1–19
Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL (2012) Visceral leishmaniasis treatment: what do we have, what do we need and how to deliver it? Int J Parasitol 2:11–19. https://doi.org/10.1016/j.ijpddr.2012.01.003
Singh N, Kumar M, Singh RK (2012) Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pac J Trop Dis 5(6):485–497. https://doi.org/10.1016/S1995-7645(12)60084-4
Hefnawy A, Berg M, Dujardin J-C, De Muylder G (2017) Exploiting knowledge on Leishmania drug resistance to support the quest for new drugs. Trends Parasitol 33(3):162–174. https://doi.org/10.1016/j.pt.2016.11.003
Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, Lopez-Velez R, Garcia-Hernandez R, Pountain AW, Mwenechanya R, Papadopoulou B (2017) Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis 11(12):e0006052. https://doi.org/10.1371/journal.pntd.0006052
Evans KJ, Kedzierski L (2012) Development of vaccines against visceral Leishmaniasis. J Trop Med 2012:892817. https://doi.org/10.1155/2012/892817
Nascimento IP, Leite LCC (2012) Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Rre 45(12):1102–1111. https://doi.org/10.1590/S0100-879X2012007500142
Akbari M, Oryan A, Hatam G (2017) Application of nanotechnology in treatment of leishmaniasis: a review. Acta Trop 172:86–90. https://doi.org/10.1016/j.actatropica.2017.04.029
Ghorbani M, Farhoudi R (2018) Leishmaniasis in humans: drug or vaccine therapy? Drug Des Dev Ther 12:25–40. https://doi.org/10.2147/DDDT.S146521
Almeida A, Machado LFM, Doro D, Nascimento FC, Damasceno L, Gazzinelli RT, Fernandes AP, Junqueira C (2018) New vaccine formulations containing a modified version of the amastigote 2 antigen and the non-virulent Trypanosoma cruzi CL-14 strain are highly antigenic and protective against Leishmania infantum challenge. Front Immunol 9:465. https://doi.org/10.3389/fimmu.2018.00465
Chávez-Fumagalli MA, Costa MA, Oliveira DM, Ramírez L, Costa LE, Duarte MC (2010) Vaccination with the Leishmania infantum ribosomal proteins induces protection in BALB/c mice against Leishmania chagasi and Leishmania amazonensis challenge. Microbes Infect 12(12–13):967–977. https://doi.org/10.1016/j.micinf.2010.06.008
Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG (2007) Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4 + T cells. Infect Immun 75:(9)4648–4654. https://doi.org/10.1128/IAI.00394-07
Rodrigues V, Cordeiro-Da-Silva A, Laforge M, Silvestre R, Estaquier J (2016) Regulation of immunity during visceral Leishmania infection. Parasite Vector 9(1):118. https://doi.org/10.1186/s13071-016-1412-x
Khadem F, Uzonna JE (2014) Immunity to visceral leishmaniasis: implications for immunotherapy. Future Microbiol 9(7):901–915. https://doi.org/10.2217/fmb.14.43
Bretscher PA (2014) On the mechanism determining the Th1/Th2 phenotype of an immune response, and its pertinence to strategies for the prevention, and treatment, of certain infectious diseases. Scand J Immunol 79(6):361–376. https://doi.org/10.1111/sji.12175
Mendonça SCF (2016) Differences in immune responses against Leishmania induced by infection and by immunization with killed parasite antigen: implications for vaccine discovery. Parasite Vector 9(1):492. https://doi.org/10.1186/s13071-016-1777-x
Nezafat N, Eslami M, Negahdaripour M, Rahbar MR, Ghasemi Y (2017) Designing an efficient multi-epitope oral vaccine against Helicobacter pylori using immunoinformatics and structural vaccinology approaches. Mol BioSyst 13(4):699–713. https://doi.org/10.1039/c6mb00772d
Validi M, Karkhah A, Prajapati VK, Nouri HR (2018) Immuno-informatics based approaches to design a novel multi epitope-based vaccine for immune response reinforcement against Leptospirosis. Mol Immunol 104:128–138. https://doi.org/10.1016/j.molimm.2018.11.005
Molero-Abraham M, Lafuente EM, Flower DR, Reche PA (2013) Selection of conserved epitopes from hepatitis C virus for pan-populational stimulation of T-cell responses. Clin Dev Immunol 2013(2013):601943. https://doi.org/10.1155/2013/601943
Solanki V, Tiwari V (2018) Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Sci Rep 8(1):9044. https://doi.org/10.1038/s41598-018-26689-7
Vakili B, Eslami M, Hatam GR, Zare B, Erfani N, Nezafat N, Ghasemi Y (2018) Immunoinformatics-aided design of a potential multi-epitope peptide vaccine against Leishmania infantum. Int J Biol Macromol 120:1127–1139. https://doi.org/10.1016/j.ijbiomac.2018.08.125
Vakili B, Nezafat N, Hatam GR, Zare B, Erfani N, Ghasemi Y (2018) Proteome-scale identification of Leishmania infantum for novel vaccine candidates: a hierarchical subtractive approach. Comput Biol Chem 72:16–25. https://doi.org/10.1016/j.compbiolchem.2017.12.008
Agallou M, Margaroni M, Athanasiou E, Toubanaki DK, Kontonikola K, Karidi K, Kammona O, Kiparissides C, Karagouni E (2017) Identification of BALB/c immune markers correlated with a partial protection to Leishmania infantum after vaccination with a rationally designed multi-epitope cysteine protease a peptide-based nanovaccine. PLoS Negl Trop Dis 11(1):e0005311. https://doi.org/10.1371/journal.pntd.0005311
Dasgupta S, Aghazadeh-Dibavar S, Bandyopadyay M (2014) The role of toll-like receptor agonists in the immunotherapy of leishmaniosis. An update and proposal for a new form of anti-leishmanial therapy. Ann Parasitol 60(2):75–82
Aathmanathan VS, Jothi N, Prajapati VK, Krishnan M (2018) Investigation of immunogenic properties of Hemolin from silkworm, Bombyx mori as carrier protein: an immunoinformatic approach. Sci Rep 8(1):6957. https://doi.org/10.1038/s41598-018-25374-z
Reed SG, Coler RN, Mondal D, Kamhawi S, Valenzuela JG (2016) Leishmania vaccine development: exploiting the host-vector-parasite interface. Expert Rev Vaccines 15(1):81–90. https://doi.org/10.1586/14760584.2016.1105135
De Brito RCF, Cardoso JMDO, Reis LES, Vieira JF, Mathias FAS, Roatt BM, Aguiar-Soares RDDO, Ruiz JC, Resende DDM, Reis AB (2018) Peptide vaccines for Leishmaniasis. Front Immunol 9(1043):1–11. https://doi.org/10.3389/fimmu.2018.01043
Hos BJ, Tondini E, Van Kasteren SI, Ossendorp F (2018) Approaches to improve chemically defined synthetic peptide vaccines. Front Immunol 9:884–884. https://doi.org/10.3389/fimmu.2018.00884
Rueckert C, Guzmán CA (2012) Vaccines: from empirical development to rational design. PLoS Pathog 8(11):e1003001. https://doi.org/10.1371/journal.ppat.1003001
Goto Y, Bogatzki LY, Bertholet S, Coler RN, Reed SG (2007) Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine 25(42):7450–7458. https://doi.org/10.1016/j.vaccine.2007.08.001
Li W, Joshi MD, Singhania S, Ramsey KH, Murthy AK (2014) Peptide vaccine: progress and challenges. Vaccines 2(3):515–536. https://doi.org/10.3390/vaccines2030515
Coelho EA, Tavares CAP, Carvalho FAA, Chaves KF, Teixeira KN, Rodrigues RC (2003) Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71(7):3988–3994. https://doi.org/10.1128/iai.71.7.3988-3994.2003
Mizbani A, Taheri T, Zahedifard F, Taslimi Y, Azizi H, Azadmanesh K, Papadopoulou B, Rafati S (2009) Recombinant Leishmania tarentolae expressing the A2 virulence gene as a novel candidate vaccine against visceral leishmaniasis. Vaccine 28(1):53–62. https://doi.org/10.1016/j.vaccine.2009.09
Resende DM, Caetano BC, Dutra MS, Penido ML, Abrantes CF, Verly RM, Resende JM, Piló-Veloso D, Rezende SA, Bruna-Romero O (2008) Epitope mapping and protective immunity elicited by adenovirus expressing the Leishmania amastigote specific A2 antigen: correlation with IFN-γ and cytolytic activity by CD8+ T cells. Vaccine 26(35):4585–4593. https://doi.org/10.1016/j.vaccine.2008.05.091
Regina-Silva S, Feres AMLT, França-Silva JC, Dias ES, Michalsky ÉM, De Andrade HM, Coelho EAF, Ribeiro GM, Fernandes AP, Machado-Coelho GLL (2016) Field randomized trial to evaluate the efficacy of the Leish-Tec® vaccine against canine visceral leishmaniasis in an endemic area of Brazil. Vaccine 34(19):2233–2239. https://doi.org/10.1016/j.vaccine.2016.03.019
Ghedin E, Zhang WW, Charest H, Sundar S, Kenney RT, Matlashewski G (1997) Antibody response against a Leishmania donovani amastigote-stage-specific protein in patients with visceral leishmaniasis. Clin Diagn Lab Immunol 4(5):530–535
Bhatia A, Daifalla NS, Jen S, Badaro R, Reed SG, Skeiky YA (1999) Cloning, characterization and serological evaluation of K9 and K26: two related hydrophilic antigens of Leishmania chagasi. Mol Biochem Parasitol 102(2):249–261
Doherty DG, Melo AM, Moreno-Olivera A, Solomos AC (2018) Activation and regulation of B cell responses by invariant natural killer T Cells. Front Immunol 9:1360. https://doi.org/10.3389/fimmu.2018.01360
Kaiko GE, Horvat JC, Beagley KW, Hansbro PM (2008) Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology 123(3):326–338. https://doi.org/10.1111/j.1365-2567.2007.02719.x
Alexander J, Brombacher F (2012) T helper1/t helper2 cells and resistance/susceptibility to leishmania infection: is this paradigm still relevant? Front Immunol 3:80–80. https://doi.org/10.3389/fimmu.2012.00080
Iborra S, Carrión J, Anderson C, Alonso C, Sacks D, Soto M (2005) Vaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c mice. Infect Immun 73(9):5842–5852. https://doi.org/10.1128/IAI.73.9.5842-5852.2005
Solano-Gallego L, Montserrat-Sangrà S, Ordeix L, Martínez-Orellana P (2016) Leishmania infantum-specific production of IFN-γ and IL-10 in stimulated blood from dogs with clinical leishmaniosis. Parasite Vector 9(1):317. https://doi.org/10.1186/s13071-016-1598-y
Wang Z-E, Reiner SL, Zheng S, Dalton DK, Locksley RM (1994) CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med 179(4):1367–1371
Gannavaram S, Bhattacharya P, Ismail N, Kaul A, Singh R, Nakhasi HL (2016) Modulation of innate immune mechanisms to enhance Leishmania vaccine-induced immunity: role of coinhibitory molecules. Front Immunol 13(7):187. https://doi.org/10.3389/fimmu.2016.00187
Gupta G, Oghumu S, Satoskar AR (2013) Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol 82:155–184. https://doi.org/10.1016/B978-0-12-407679-2.00005-3
Liew F, Parkinson C, Millott S, Severn A, Carrier M (1990) Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology 69(4):570
Sjölander A, Baldwin TM, Curtis JM, Handman E (1998) Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J Immunol 160(8):3949–3957
Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S (2005) Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and-resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1-and Th2-like responses in visceral leishmaniasis. J Immunol 174(11):7160–7171. https://doi.org/10.4049/jimmunol.174.11.7160
Roberts MT, Stober CB, Mckenzie AN, Blackwell JM (2005) Interleukin-4 (IL-4) and IL-10 collude in vaccine failure for novel exacerbatory antigens in murine Leishmania major infection. Infect Immun 73(11):7620–7628
Hurdayal R, Brombacher F (2014) The role of IL-4 and IL-13 in cutaneous Leishmaniasis. Immunol Lett 161(2):179–183. https://doi.org/10.1016/j.imlet.2013.12.022
Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP (2001) IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol 31(10):2848–2856
Mcgeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, Mcclanahan T, Cua DJ (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8(12):1390–1397. https://doi.org/10.1038/ni1539
Biedermann T, Zimmermann S, Himmelrich H, Gumy A, Egeter O, Sakrauski AK, Seegmuller I, Voigt H, Launois P, Levine AD, Wagner H, Heeg K, Louis JA, Rocken M (2001) IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol 2(11):1054–1060. https://doi.org/10.1038/ni725
Vouldoukis I, Becherel PA, Riveros-Moreno V, Arock M, Da Silva O, Debre P, Mazier D, Mossalayi MD (1997) Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur J Immunol 27(4):860–865. https://doi.org/10.1002/eji.1830270409
Nylén S, Gautam S (2010) Immunological perspectives of leishmaniasis. J Glob Infect Dis 2(2):135–146. https://doi.org/10.4103/0974-777X.62876
Ghosh A, Zhang WW, Matlashewski G (2001) Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine 20(1–2):59–66
Darabi H, Eravani D, Sanos S, Kaye P, Taghikhani M, Jamshidi S (2005) Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine 23(28):3716–3725
Rafati S, Zahedifard F, Nazgouee F (2006) Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine 24(12):2169–2175
Mutiso JM, Macharia JC, Gicheru MM (2010) A review of adjuvants for Leishmania vaccine candidates. Biomed Res 24(1):16–25. https://doi.org/10.1016/S1674-8301(10)60004-8
Gnjatic S, Sawhney NB, Bhardwaj N (2010) Toll-like receptor agonists: are they good adjuvants? Cancer J (Sudbury, Mass) 16(4):382–391. https://doi.org/10.1097/PPO.0b013e3181eaca65
Li Q, Guo Z (2018) Recent advances in toll like receptor-targeting glycoconjugate vaccines. Molecules. https://doi.org/10.3390/molecules23071583
Reed SG, Hsu F-C, Carter D, Orr MT (2016) The science of vaccine adjuvants: advances in TLR4 ligand adjuvants. Curr Opin Immunol 41:85–90
Kim JS, Kim WS, Choi HG, Jang B, Lee K, Park JH, Kim HJ, Cho SN, Shin SJ (2013) Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J Leukoc Biol 94(4):733–749. https://doi.org/10.1189/jlb.0912435
Choi HG, Kim WS, Back YW, Kim H, Kwon KW, Kim JS, Shin SJ, Kim HJ (2015) Mycobacterium tuberculosis RpfE promotes simultaneous Th1- and Th17-type T-cell immunity via TLR4-dependent maturation of dendritic cells. Eur J Immunol 45(7):1957–1971. https://doi.org/10.1002/eji.201445329
Acknowledgements
This study was supported by a Grant agreement no. 13435 from Shiraz University of Medical Sciences, Shiraz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Edited by Christian Bogdan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
430_2019_640_MOESM1_ESM.jpg
Fig. S1. Schematic diagram of the designed multi-epitope peptide vaccine. The sequence consists of 437 residues; out of which, the first 144 residues are related to the RpfE adjuvant followed by the nine immunodominant epitopes from SMT, LSAP, LiHy, H1 linked together by AAYKK and GSGSGS linkers. The second adjuvant is RpfB with 80 amino acids that is located at the other end of the construct. SMT: sterol 24-c-methyltransferase, LSAP: Leishmania-specific antigenic protein, LiHy: Leishmania-specific hypothetical protein, H1: Histone H1
Fig. S2
. The chimera sequence of the final peptide construct
Rights and permissions
About this article
Cite this article
Vakili, B., Nezafat, N., Zare, B. et al. A new multi-epitope peptide vaccine induces immune responses and protection against Leishmania infantum in BALB/c mice. Med Microbiol Immunol 209, 69–79 (2020). https://doi.org/10.1007/s00430-019-00640-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00640-7