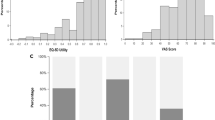
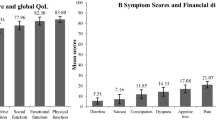
Abstract
Purpose
Explore patient-reported outcomes (PROs), including health-related quality of life (HRQoL), satisfaction with therapy, impact of the therapy on work and daily life, and concerns related to the therapy and identify variables associated with PROs in persons with chronic myeloid leukemia (CML) receiving tyrosine kinase inhibitors (TKIs).
Methods
Across-sectional questionnaire was distributed to adults with chronic phase CML and answered anonymously. SF-36 Health Survey was used to measure HRQoL. Our focus was on the physical component summary (PCS) and mental component summary (MCS) components.
Results
Data from 819 respondents receiving TKI-therapy ≥3 months and achieving a complete cytogenetic response were analyzed. Median age was 42 years (range 18–88 years). 652 (80%) were receiving imatinib. Median TKI-therapy duration was 36 months (range 3–178 months). 629 (77%) paid some or all of their TKI costs. In multivariate analyses, female sex, increasing age, lower education level, increasing co-morbidities, concomitant medication, ≥3 symptoms, moderate or severe symptom, switch from imatinib to a second-generation TKI, and higher annual out-of-pocket expense of TKI were significantly associated with lower PCS and/or MCS. However, TKI-therapy duration 3–7 years was significantly associated with better well-being. Higher PCS or MCS score was significantly associated with higher satisfaction level with TKI-therapy and less impact of TKI-therapy on subject’s daily life and work. In addition, adverse impact on daily life and work was significantly associated with more interests in TKI-therapy-related issues.
Conclusions
Social-economic and clinical variables were significantly associated with PROs in persons with CML receiving TKI-therapy.


Similar content being viewed by others
References
Aziz Z, Iqbal J, Aaqib M, Akram M, Saeed A (2011) Assessment of quality of life with imatinibmesylate as first-line treatment in chronic phase-chronic myeloid leukemia. Leuk Lymphoma 52(6):1017–1023
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P et al (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34(6):557–565
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM (2016) Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol 34(24):2851–2857
Breccia M, Graffigna G, Galimberti S, Iurlo A, Pungolino E, Pizzuti M et al (2016) Personal history and quality of life in chronic myeloid leukemia patients: a cross-sectional study using narrative medicine and quantitative analysis. Support Care Cancer 24(11):4487–4493
Cortes JE, Lipton JH, Miller CB, Busque L, Akard LP, Pinilla-Ibarz J et al (2016) Evaluating the impact of a switch to nilotinib on imatinib-related chronic low-grade adverse events in patients with CML-CP: the ENRICH study. Clin Lymphoma Myeloma Leuk 16(5):286–296
Efficace F, Baccarani M, Breccia M, Alimena G, Rosti G, Cottone F et al (2011) GIMEMA. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 118(17):4554–4560
Efficace F, Breccia M, Saussele S, Kossak-Roth U, Cardoni A, Caocci G et al (2012) Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC Quality of Life Group. Ann Hematol 91(9):1371–1381
Efficace F, Baccarani M, Breccia M, Cottone F, Alimena G, Deliliers GL et al (2013) Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 27(7):1511–1519
Efficace F, Baccarani M, Breccia M, Saussele S, Abel G, Caocci G et al (2014) International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res 23(3):825–836
Efficace F, Rosti G, Breccia M, Cottone F, Giesinger JM, Stagno F et al (2016a) The impact of comorbidity on health-related quality of life in elderly patients with chronic myeloid leukemia. Ann Hematol 95(2):211–219
Efficace F, Breccia M, Cottone F, Okumura I, Doro M, Riccardi F et al (2016b) Psychological well-being and social support in chronic myeloid leukemia patients receiving lifelong targeted therapies. Support Care Cancer 24(12):4887–4894
Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C et al (2011) Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 103(7):553–561
Gu´erinA, Chen L, Ionescu-Ittu R, Marynchenko M, Nitulescu R, Hiscock R (2014) Impact of low-grade adverse events on health-related quality of life in adult patients receiving imatinib or nilotinib for newly diagnosed Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase. Curr Med Res 30(11):2317–2328
Guest JF, Gray EJ, Szczudlo T, Magestro M (2014) Utility values for specific chronic myeloid leukemia chronic phase health states from the general public in the United Kingdom. Leuk Lymphoma 55(8):1870–1875
Gunnarsson N, Sandin F, Höglund M, Stenke L, Björkholm M, Lambe M et al (2016) Population-based assessment of chronic myeloid leukemia in Sweden: striking increase in survival and prevalence. Eur J Haematol 97(4):387–392
Hahn EA, Glendenning GA, Sorensen MV, Hudgens SA, Druker BJ, Guilhot F et al (2003) IRIS Investigators. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low-dose cytarabine: results from the IRIS Study. J Clin Oncol 21(11):2138–2146
Hamerschlak N, de Souza C, Cornacchioni AL, Pasquini R, Tabak D, Spector N, Steagall M (2014) Quality of life of chronic myeloid leukemia patients in Brazil: ability to work as a key factor. Support Care Cancer 22(8):2113–2118
Hirji I, Gupta S, Goren A, Chirovsky DR, Moadel AB, Olavarria E et al (2013) Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient’s perspective. Health Qual Life Outcomes 11:167
Huang X, Cortes J, Kantarjian H (2012) Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer 118(12):3123–3127
Jain P, Das VN, Ranjan A, Chaudhary R, Pandey K (2013) Comparative study for the efficacy, safety and quality of life in patients of chronic myeloid leukemia treated with Imatinib or Hydroxyurea. J Res Pharm Pract 2(4):156–161
Kekäle M, Peltoniemi M, Airaksinen M (2015) Patient-reported adverse drug reactions and their influence on adherence and quality of life of chronic myeloid leukemia patients on per oral tyrosine kinase inhibitor treatment. Patient Prefer Adherence 9:1733–1740
Lauseker M, Gerlach R, Tauscher M, Hasford J (2016) Improved survival boosts the prevalence of chronic myeloid leukemia: predictions from a population-based study. J Cancer Res Clin Oncol 142(7):1441–1447
Li L, Wang HM, Shen Y (2003) Chinese SF-36 Health Survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health 57:259–263
Mesa RA, Miller CB, Thyne M, Mangan J, Goldberger S, Fazal S et al (2016) Differences in treatment goals and perception of symptom burden between patients with myeloproliferative neoplasms (MPNs) and hematologists/oncologists in the United States: findings from the MPN landmark survey. Cancer. doi:10.1002/cncr.30325 (Epub ahead of print] PubMed PMID: 27690182)
Mo XD, Jiang Q, Xu LP, Liu DH, Liu KY, Jiang B et al (2014) Health-related quality of life of patients with newly diagnosed chronic myeloid leukemia treated with allogeneic hematopoietic SCT versus imatinib. Bone Marrow Transplant 49(4):576–580
National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology: Chronic Myelogenous Leukemia Version 1 (2016) Fort Washington PA. National Comprehensive Care Network. http://www.nccn.Org/professionals/physician_gls/pdf/cml.pdf. Accessed 26 March 2016
Park JS, Lee SE, Jeong SH, Jang EJ, Choi MY, Kim HJ et al (2016) Change of health-related profiles after Imatinib cessation in chronic phase chronic myeloid leukemia patients. Leuk Lymphoma 57(2):341–347
Phillips KM, Pinilla-Ibarz J, Sotomayor E, Lee MR, Jim HS, Small BJ et al (2013) Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer 21(4):1097–1103
Sasaki K, Strom SS, O’Brien S, Jabbour E, Ravandi F, Konopleva M et al (2015) Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol 2(5):e186–93
Trask PC, Cella D, Besson N, Kelly V, Masszi T, Kim DW (2012) Health-related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemia. Leuk Res 36(4):438–442
Trask PC, CellaD, Powell C, Reisman A, Whiteley J, Kelly V (2013) Health-related quality of life in chronic myeloid leukemia. Leuk Res 37(1):9–13
Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A (1995) Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care 33:AS264–A79
Whiteley J, ReismanA, Shapiro M, Cortes J, Cella D (2016) Health-related quality of life during bosutinib (SKI-606) therapy in patients with advanced chronic myeloid leukemia after imatinib failure. Curr Med Res Opin 2(8):1325–1334
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (No. 81370637).
Conflict of interest
Author RPG is a part-time employee of Celgene Corporation, Summit, NJ, USA. The remaining authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Because the survey used anonymous questionnaires, the Ethics Committee of Peoples Hospital determined informed consent of participants was not required.
Additional information
Q. Jiang and H.-B. Wang contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Q., Wang, HB., Yu, L. et al. Variables associated with patient-reported outcomes in persons with chronic myeloid leukemia receiving tyrosine kinase-inhibitor therapy. J Cancer Res Clin Oncol 143, 1013–1022 (2017). https://doi.org/10.1007/s00432-017-2353-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2353-2