Abstract
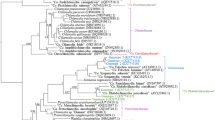
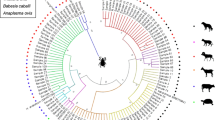
Ticks are well known to be important vectors for a wide range of bacteria, viruses and protozoa affecting human and animal health. Ixodid ticks are widely distributed in Sardinia, and an increasing number of tick-borne bacteria have been documented in the island. A growing number of evidence are supporting the hypothesis of alternative transmission routes for chlamydial bacteria such as the involvement of vectors. This study was conducted to provide possible molecular detection of members belonging to the Chlamydiales order in Sardinian ticks and to update information concerning the presence of new ectoparasite-borne bacteria in ticks collected from domestic and wild hosts in a typical Mediterranean environment. A total of 378 ticks were individually screened with a pan-Chlamydiales specific primers targeting the 16S rRNA gene. Chlamydiales DNA was detected in 28% of the total ticks analyzed. The analyses of sequences highlighted that Rhipicephalus sanguineus sensu lato, Rhipicephalus bursa, Rhipicephalus annulatus, Haemaphysalis sulcata, Haemaphysalis punctata and Dermacentor marginatus ticks exhibited DNA of Chlamydiaceae and Parachlamydiaceae members. Our results revealed that DNA of zoonotic microorganisms such as C. psittaci, C. abortus and the emerging pathogen Parachlamydia acanthamoebae are present in Sardinian ticks. Since routes of Chlamydia transmission are yet to be fully defined, the role of ticks as possible vectors for Chlamydiales remains the most challenging and interesting question to be addressed in future research. Continued monitoring of these pathogens in tick vectors is needed to provide strategies for controlling of possible chlamydial infections and disease outbreaks in the island.


Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, LipmanDJ (1990) Basic Local Alignment Search Tool. J Mol Biol 215(3):403–410
Baud D, Greub G (2011) Intracellular bacteria and adverse pregnancy outcomes. Clin Microbiol Infect 17(9):1312–1322. https://doi.org/10.1111/j.1469-0691.2011.03604.x
Burnard D, Weaver H, Gillett A, Loader J, Flanagan C, Polkinghorne A (2017) Novel Chlamydiales genotypes identified in ticks from Australian wildlife. Parasit Vectors 10(1):46. https://doi.org/10.1186/s13071-017-1994-y
Caldwell HD, Belden EL (1973) Studies of the role of Dermacentor occidentalis in the transmission of bovine chlamydial abortion. Infect Immun 7(2):147–151
Chisu V, Porcu R, Tanda A, Masala G (2013) First isolation and characterization of Chlamydophila abortus from abortion tissues of sheep in Sardinia, Italy. Vet Ital 49(4):331–334. https://doi.org/10.12834/VetIt.1303.10
Chisu V, Masala G, Foxi C, Socolovschi C, Raoult D, Parola P (2014) Rickettsia conorii israelensis in Rhipicephalus sanguineus ticks, Sardinia, Italy. Ticks Tick Borne Dis 5(4):446–448. https://doi.org/10.1016/j.ttbdis.2014.02.003
Chisu V, Leulmi H, Masala G, Piredda M, Foxi C, Parola P (2017) Detection of Rickettsia hoogstraalii, Rickettsia helvetica, Rickettsia massiliae, Rickettsia slovaca and Rickettsia aeschlimannii in ticks from Sardinia, Italy. Ticks Tick Borne Dis 8(3):347–352. https://doi.org/10.1016/j.ttbdis.2016.12.007
Croxatto A, Rieille N, Kernif T, Bitam I, Aeby S, Péter O, Greub G (2014) Presence of Chlamydiales DNA in ticks and fleas suggests that ticks are carriers of Chlamydiae. Ticks Tick Borne Dis 5(4):359–365. https://doi.org/10.1016/j.ttbdis.2013.11.009
Doosti A, Arshi A (2011) Determination of the prevalence of Chlamydia psittaci by PCR in Iranian pigeons. Int J Biol 3(4):79–82
Essig A, Longbottom D (2015) Chlamydia abortus: new aspects of infectious abortion in sheep and potential risk for pregnant women. Curr Clin Microbiol Rep 2(1):22–34. https://doi.org/10.1007/s40588-015-0014-2
Everett KDE (2000) Chlamydia and Chlamydiales: more than meets the eye. Vet Microbiol 75(2):109–126. https://doi.org/10.1016/S0378-1135(00)00213-3
Everett KD, Bush RM, Andersen AA (1999) Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. Nov. and Simkaniaceae fam. Nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol 49:415–440
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser (Oxford) 41:95–98
Hokynar K, Sormunen JJ, Vesterinen EJ, Partio EK, Lilley T, Timonen V, Panelius J, Ranki A, Puolakkainen M (2016) Chlamydia-like organisms (CLOs) in Finnish Ixodes ricinus ticks and human skin. Microorganisms 18:4
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3–14
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120. https://doi.org/10.1007/BF01731581
Lamoth F, Greub G (2010) Amoebal pathogens as emerging causal agents of pneumonia. FEMS Microbiol Rev 34(3):260–280. https://doi.org/10.1111/j.1574-6976.2009.00207.x
Larkin MA, Blackshields G, Brown NP, Chenna R , McGettigan PA, McWilliam H , Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and clustal x version 2.0. Bioinformatics 23(21):2947–2948
Leulmi H, Aouadi A, Bitam I, Bessas A, Benakhla A, Raoult D, Parola P (2016) Detection of Bartonella tamiae, Coxiella burnetii and Rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasit Vectors 20:9–27
Longbottom D, Coulter LJ (2003) Animal chlamydioses and zoonotic implications. J Comp Pathol 128(4):217–244. https://doi.org/10.1053/jcpa.2002.0629
Longbottom D, Russell M, Dunbar SM, Jones GE, Herring AJ (1998) Molecular cloning and characterization of the genes coding for the highly immunogenic cluster of 90-kilodalton envelope proteins from the Chlamydia psittaci subtype that causes abortion in sheep. Infect Immun 66(4):1317–1324
Manilla G (1998) Acari, Ixodida. Fauna d’Italia 36. Edizioni Calderini, Bologna
Masala G, Porcu R, Sanna G, Tanda A, Tola S (2005) Role of Chlamydophila abortus in ovine and caprine abortion in Sardinia, Italy. Vet Res Commun 1:117–123
Masala G, Porcu R, Daga C, Denti S, Canu G, Patta C, Tola S (2007) Detection of pathogens in ovine and caprine abortion samples from Sardinia, Italy, by PCR. J Vet Diagn Investig 19:96–98
Masala G, Chisu V, Foxi C, Socolovschi C, Raoult D, Parola P (2012) First detection of Ehrlichia canis in Rhipicephalus bursa ticks in Sardinia, Italy. Ticks Tick Borne Dis 3(5-6):396–397. https://doi.org/10.1016/j.ttbdis.2012.10.006
McKercher DG, Wada EM, Ault SK, Theis JH (1980) Preliminary studies on transmission of Chlamydia to cattle by ticks (Ornithodoros coriaceus). Am J Vet Res 41(6):922–924
Nakao R, Abe T, Nijhof AM, Yamamoto S, Jongejan F, Ikemura T, Sugimoto C (2013) A novel approach, based on BLSOMs (batch learning self-organizing maps), to the microbiome analysis of ticks. ISME J 7:1003–1015
Omsland A, Sixt BS, Horn M, Hackstadt T (2014) Chlamydial metabolism revisited: interspecies metabolic variability and developmental stage-specific physiologic activities. FEMS Microbiol Rev 38(4):779–801. https://doi.org/10.1111/1574-6976.12059
Opota O, Jaton K, Branley J, Vanrompay D, Erard V, Borel N, Longbottom D, Greub G (2015) Improving the molecular diagnosis of Chlamydia psittaci and Chlamydia abortus infection with a species-specific duplex real-time PCR. J Med Microbiol 64(10):1174–1185. https://doi.org/10.1099/jmm.0.000139
Pilloux L, Aeby S, Gaümann R, Burri C, Beuret C, Greub G (2015) High prevalence and diversity of Chlamydiales DNA within Ixodes ricinus ticks suggest a role of ticks as reservoir and vectors of chlamydia-related bacteria. Appl Environ Microbiol 81(23):8177–8182. https://doi.org/10.1128/AEM.02183-15
Rehn M, Ringberg H, Runehagen A, Herrmann B, Olsen B, Petersson AC, Hjertqvist M, Kühlmann-Berenzon S, Wallensten A (2013) Unusual increase of psittacosis in southern Sweden linked to wild bird exposure, January to April 2013. Euro Surveill 18:20478
Salvatore D, Galuppi R, Aureli S, Tampieri MP, Di Francesco A (2016) Detection of Chlamydiales DNA in questing ticks. Vet Rec 179:48
Satta G, Chisu V, Cabras P, Fois F, Masala G (2011) Pathogens and symbionts in ticks: a survey on tick species distribution and presence of tick-transmitted micro-organisms in Sardinia, Italy. J Med Microbiol 60:63–68
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Taylor-Brown A, Vaughan L, Greub G, Timms P, Polkinghorne A (2015) Twenty years of research into Chlamydia-like organisms: a revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. Pathog Dis 73(1):1–15. https://doi.org/10.1093/femspd/ftu009
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animal were followed.
Additional information
Section Editor: Domenico Otranto
Rights and permissions
About this article
Cite this article
Chisu, V., Foxi, C., Tanda, A. et al. Molecular evidence of Chlamydiales in ticks from wild and domestic hosts in Sardinia, Italy. Parasitol Res 117, 981–987 (2018). https://doi.org/10.1007/s00436-018-5772-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5772-3