Abstract
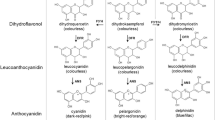
Pink-flowered gentian plants (Gentiana scabra) have been bred from spontaneous mutations of blue-flowered gentian plants, but the formation mechanism(s) is unknown so far. To investigate the process, two independent pink-flowered gentian plant lines were analyzed by a molecular biological approach. HPLC analysis showed that petals of the blue-flowered cultivar contained a small amount of cyanidin derivatives and major delphinidin derivatives, whereas pink petals had only a small amount of cyanidin derivatives. To find the causal factor(s) of this change, we focused on flavonoid 3′,5′-hydroxylase (F3′,5′H), which is a key enzyme for delphinidin biosynthesis in the flavonoid biosynthetic pathway. Molecular analyses confirmed that the loss of delphinidin synthesis could be attributed to the insertions of different transposable elements in the F3′,5′H gene in each independent pink-flowered gentian plant. Sequence analysis showed that these transposable elements were classified into an hAT superfamily and terminal-repeat retrotransposon in miniature (TRIM), by which normal F3′,5′H transcripts were interrupted. Southern blot analysis indicated that they belong to high copy number elements and are also found in a related gentian species (G. triflora). These results suggest that the transposable elements inserted in F3′,5′H are the source of the mutations and may also play a substantial role in the genomic evolution of the genus Gentiana.






Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Brown JJ, Mattes MG, O’Reilly C, Shepherd NS (1989) Molecular characterization of rDt, a maize transposon of the “Dotted” controlling element system. Mol Gen Genet 215:239–244
Carpenter R, Martin C, Coen ES (1987) Comparison of genetic behaviour of the transposable element Tam3 at two unlinked pigment loci in Antirrhinum majus. Mol Gen Genet 207:82–89
Feschotte C, Jiang N, Wessler SR (2002) Plant transposable elements: where genetics meets genomics. Nat Rev Genet 3:329–341
Fujiwara H, Tanaka Y, Yonekura-Sakakibara K, Fukuchi-Mizutani M, Nakao M, Fukui Y, Yamaguchi M, Ashikari T, Kusumi T (1998) cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J 16:421–431
Fukuchi-Mizutani M, Okuhara H, Fukui Y, Nakao M, Katsumoto Y, Yonekura-Sakakibara K, Kusumi T, Hase T, Tanaka Y (2003) Biochemical and molecular characterization of a novel UDP-glucose:anthocyanin 3′-O-glucosyltransferase, a key enzyme for blue anthocyanin biosynthesis, from gentian. Plant Physiol 132:1652–1663
Gerats AG, Huits H, Vrijlandt E, Marana C, Souer E, Beld M (1990) Molecular characterization of a nonautonomous transposable element (dTph1) of petunia. Plant Cell 2:1121–1128
Goto T, Kondo T, Tamura H, Imagawa H, Iino H, Takeda K (1982) Structure of gentiodelphin, an acylated anthocyanin isolated from Gentiana makinori, that is stable in dilute aqueous solution. Tetrahedron Lett 23:3695–3698
Graig NJ (2002) Mobile DNA. In: Craig NJ, Craigle R, Gellert M, Lambowitz AM (eds) Mobile DNA II. American Society for Microbiology Press, Washington DC, pp 3–11
Grandbastien MA, Spielmann A, Caboche M (1989) Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature 337:376–380
Grappin P, Audeon C, Chupeau MC, Grandbastien MA (1996) Molecular and functional characterization of Slide, an Ac-like autonomous transposable element from tobacco. Mol Gen Genet 252:386–397
Hashida S, Kitamura K, Mikami T, Kishima Y (2003) Temperature shift coordinately changes the activity and the methylation state of transposon Tam3 in Antirrhinum majus. Plant Physiol 132:1207–1216
Hehl R, Nacken WK, Krause A, Saedler H, Sommer H (1991) Structural analysis of Tam3, a transposable element from Antirrhinum majus, reveals homologies to the Ac element from maize. Plant Mol Biol 16:369–371
Henk AD, Warren RF, Innes RW (1999) A new Ac-like transposon of Arabidopsis is associated with a deletion of the RPS5 disease resistance gene. Genetics 151:1581–1589
Hirochika H, Fukuchi A, Kikuchi F (1992) Retrotransposon families in rice. Mol Gen Genet 233:209–216
Holton TA, Brugliera F, Lester DR, Tanaka Y, Hyland CD, Menting JG, Lu CY, Farcy E, Stevenson TW, Cornish EC (1993) Cloning and expression of cytochrome P450 genes controlling flower colour. Nature 366:276–279
Holten TA, Cornish EC (1995) Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7:1071–1083
Hoshino A, Morita Y, Choi JD, Saito N, Toki K, Tanaka Y, Iida S (2003) Spontaneous mutations of the flavonoid 3′-hydroxylase gene conferring reddish flowers in the three morning glory species. Plant Cell Physiol 44:990–1001
Hosokawa K, Fukushi E, Kawabata J, Fujii C, Ito T, Yamamura S (1995) Three acylated cyanidin glucosides in pink flowers of Gentiana. Phytochemistry 40:941–944
Hosokawa K, Fukushi E, Kawabata J, Fujii C, Ito T, Yamamura S (1997) Seven acylated anthocyanins in blue flowers of Gentiana. Phytochemistry 45:167–171
Jiang N, Bao Z, Zhang X, Hirochika H, Eddy SR, McCouch SR, Wessler SR (2003) An active DNA transposon family in rice. Nature 421:163–167
Kikuchi K, Terauchi K, Wada M, Hirano H (2003) The plant MITE mPing is mobilized in anther culture. Nature 421:167–170
Kobayashi H, Oikawa Y, Koiwa H, Yamamura S (1998) Flower-specific expression directed by the promoter of a chalcone synthase gene from Gentiana triflora in Petunia hybrida. Plant Sci 131:173–180
Koes R, Verweij W, Quattrocchio F (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10:236–242
Kreahling J, Graveley BR (2004) The origins and implications of alternative splicing. Trends Genet 20:1–4
Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet 33:479–532
Kunze R, Starlinger P (1989) The putative transposase of transposable element Ac from Zea mays L. interacts with subterminal sequences of Ac. EMBO J 8:3177–3185
Kunze R, Weil CF (2002) The hAT and CACTA superfamilies of plant transposons. In: Craig NJ, Craigle R, Gellert M, Lambowitz AM (eds) Mobile DNA II. American Society for Microbiology Press, Washington DC, pp 565–610
Liu D, Crawford NM (1998) Characterization of the germinal and somatic activity of the Arabidopsis transposable element Tag1. Genetics 148:445–456
Marchler-Bauer A, Bryant SH (2004) CD-Search: protein domain annotations on the fly. Nucleic Acids Res 32:W327–331
Miura A, Yonebayashi S, Watanabe K, Toyama T, Shimada H, Kakutani T (2001) Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 411:212–214
Muller-Neumann M, Yoder JI, Starlinger P (1984) The DNA sequence of the transposable element Ac of Zea mays L. Mol Gen Genet 198:19–24
Mol J, Grotewold E, Koes R (1998) How genes paint flowers and seeds. Trends Plant Sci 3:212–216
Nakatsuka T, Nishihara M, Mishiba K, Yamamura S (2005) Temporal expression of flavonoid biosynthesis-related genes regulates flower pigmentation in gentian plants. Plant Sci 168:1309–1318
Nakazaki T, Okumoto Y, Horibata A, Yamahira S, Teraishi M, Nishida H, Inoue H, Tanisaka T (2003) Mobilization of a transposon in the rice genome. Nature 421:170–172
Page RDM (1996) TREEVIEW: an application to display phylogenic trees on personal computers. Comp Appl Biosci 12:357–358
Pereira A, Cuypers H, Gierl A, Schwarz-Sommer Z, Saedler H (1986) Molecular analysis of the En/Spm transposable element system of Zea mays. EMBO J 5:835–841
SanMiguel P, Bennetzen JL (1998) Evidence that a recent increase in maize genome size was caused by the massive amplification of intergene retrotransposons. Ann Bot 82:37–44
SanMiguel P, Tikhonov A, Jin YK, Motchoulskaia N, Zakharov D, Melake-Berhan A, Springer PS, Edwards KJ, Lee M, Avramova Z, Bennetzen JL (1996) Nested retrotransposons in the intergenic regions of the maize genome. Science 274:765–768
Snowden KC, Napoli CA (1998) Psl: a novel Spm-like transposable element from Petunia hybrida. Plant J 14:43–54
Sorek R, Ast G, Graur D (2002) Alu-containing exons are alternatively spliced. Genome Res 12:1060–1067
Tanaka Y, Yonekura K, Fukuchi-Mizutani M, Fukui Y, Fujiwara H, Ashikari T, Kusumi T (1996) Molecular and biochemical characterization of three anthocyanin synthetic enzymes from Gentiana triflora. Plant Cell Physiol 37:711–716
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
van Houwelingen A, Souer E, Spelt K, Kloos D, Mol J, Koes R (1998) Analysis of flower pigmentation mutants generated by random transposon mutagenesis in Petunia hybrida. Plant J 13:39–50
Varagona MJ, Purugganan M, Wessler SR (1992) Alternative splicing induced by insertion of retrotransposons into the maize waxy gene. Plant Cell 4:811–820
Vicient CM, Kalendar R, Anamthawat-Jonsson K, Schulman AH (1999) Structure, functionality, and evolution of the BARE-1 retrotransposon of barley. Genetica 107:53–63
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Witte CP, Le QH, Bureau T, Kumar A (2001) Terminal-repeat retrotransposons in miniature (TRIM) are involved in restructuring plant genomes. Proc Natl Acad Sci USA 98:13778–13783
Witte CP, Tiller S, Isidore E, Davies HV, Taylor MA (2005) Analysis of two alleles of the urease gene from potato: polymorphisms, expression, and extensive alternative splicing of the corresponding mRNA. J Exp Bot 56:91–99
Acknowledgments
We thank Mr. Katsuo Kodama (Iwate Agriculture Research Center, Japan) for providing the gentian materials. We also thank Dr. Yoshihiro Ozeki (Tokyo University of Agriculture and Technology, Japan) and Dr. Toshio Aoki (Nihon University, Japan) for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.-A. Grandbastien
Nucleotide sequence data reported are available in the DDBJ/EMBL/GenBank databases under accession numbers AB222604 (Gentiana scabra F3’,5’H genome sequence), AB222605 (dTgs1), and AB222606 (GsTRIM1).
Rights and permissions
About this article
Cite this article
Nakatsuka, T., Nishihara, M., Mishiba, K. et al. Two different transposable elements inserted in flavonoid 3′,5′-hydroxylase gene contribute to pink flower coloration in Gentiana scabra . Mol Genet Genomics 275, 231–241 (2006). https://doi.org/10.1007/s00438-005-0083-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-0083-7