Abstract
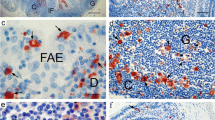
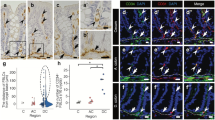
Several types of macrophages have been reported in the intestinal mucosa, but their histological localization remains ambiguous. Here, we obtained detailed information about ultrastructural and phenotypical diversity of macrophage-like cells (MLCs) in the rat ileal mucosa using immunofluorescent analysis and serial block-face scanning electron microscopy (SBF-SEM). The results revealed that the cells immunopositive for CD68, the pan-macrophage marker, included CD163−CD4+, CD163+CD4+, and CD163−CD4− cells in the lamina propria (LP) of the intestinal villus and around the crypt. CD68+CD4+CD163− cells seemed to be preferentially localized in the intestinal villus, whereas CD68+CD163+CD4+ cells were frequently localized around the crypt. SBF-SEM analysis identified three types of MLCs in the ileal mucosa, which were tentatively named types I–III MLC based on aspects of the 3D-ultrastructure, such as the localization, quantity of lysosomes, endoplasmic reticulum, and exoplasm. Type I and II MLCs were localized in the villous LP, while type III MLCs were localized around the crypt, although type II MLCs were a minor population. All three MLC types extended their cellular processes into the epithelium, with type I MLCs showing the greatest abundance of extended processes. Type I MLCs in the upper portion of the intestinal villus showed a higher level of attachment to intraepithelial lymphocytes (IELs) compared to type III MLCs around the crypt. These findings suggest that macrophages of the rat ileal mucosa differed by region along the longitudinal axis of the villous tip-crypt from the perspective of ultrastructure, cellular composition, localization, and interactions with IELs.








Similar content being viewed by others
References
Arai M, Mantani Y, Nakanishi S, Haruta T, Nishida M, Yuasa H, Yokoyama T, Hoshi N, Kitagawa H (2020) Morphological and phenotypical diversity of eosinophils in the rat ileum. Cell Tissue Res 381:439–450
Bekiaris V, Persson EK, Agace WW (2014) Intestinal dendritic cells in the regulation of mucosal immunity. Immunol Rev 260:86–101
Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M (2009) Origin of the lamina propria dendritic cell network. Immunity 31:513–525
Briggman KL, Helmstaedter M, Denk W (2011) Wiring specificity in the direction-selectivity circuit of the retina. Nature 471:183–188
Bujko A, Atlasy N, Landsverk OJB, Richter L, Yaqub S, Horneland R, Øyen O, Aandahl EM, Aabakken L, Stunnenberg HG, Bækkevold ES, Jahnsen FL (2018) Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med 215:441–458
Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SW (2013) Intestinal CD103(-) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol 6:104–113
Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O’Keeffe M, Liao G, Karp CL, Kweon MN, Sharpe AH, Bhan A, Terhorst C, Reinecker HC (2013) Circulatory antigen processing by mucosal dendritic cells controls CD8+ T cell activation. Immunity 38:153–165
Chapuy L, Bsat M, Sarkizova S, Rubio M, Therrien A, Wassef E, Bouin M, Orlicka K, Weber A, Hacohen N, Villani AC, Sarfati M (2019) Two distinct colonic CD14+subsets characterized by single- cell RNA profiling in Crohn’s disease. Mucosal Immunol 12:703–719
Chieppa M, Rescigno M, Huang AY, Germain RN (2006) Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 203:2841–2852
Cummings RJ, Barbet G, Bongers G, Hartmann BM, Gettler K, Muniz L, Furtado GC, Cho J, Lira SA, Blander JM (2016) Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539:565–569
Deane HW (1964) Some electron microscopic observations on the lamina propria of the gut, with comments on the close association of macrophages, plasma cells, and eosinophils. Anat Rec 149:453–473
Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, Karp CL, Pulendran B (2011) Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol 187:733–747
Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 8:1086–1094
De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de Casterlé I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, Boeckxstaens G (2018) Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175:400–415
Dijkstra CD, Döpp EA, Joling P, Kraal G (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv Exp Med Biol 186:409–419
Eustaquio T, Wang C, Dugard CK, George NI, Liu F, Slikker WJ, Paule MG, Howard PC, Paredes AM (2018) Electron microscopy techniques employed to explore mitochondrial defects in the developing rat brain following ketamine treatment. Exp Cell Res 373:164–170
Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G (2013) Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38:581–595
Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D (2016) Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164:378–391
Iwanaga T, Hoshi O, Han H, Takahashi-Iwanaga H, Uchiyama Y, Fujita T (1994) Lamina propria macrophages involved in cell death (apoptosis) of enterocytes in the small intestine of rats. Arch Histol Cytol 57:267–276
Kang B, Alvarado LJ, Kim T, Lehmann ML, Cho H, He J, Li P, Kim BH, Larochelle A, Kelsall BL (2020) Commensal microbiota drive the functional diversification of colon macrophages. Mucosal Immunol 13:216–229
Lich JD, Elliott JF, Blum JS (2000) Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med 191:1513–1524
Mantani Y, Haruta T, Nishida M, Yokoyama T, Hoshi N, Kitagawa H (2019) Three-dimensional analysis of fibroblast-like cells in the lamina propria of the rat ileum using serial block-face scanning electron microscopy. J Vet Med Sci 81:454–465
Mantani Y, Nishida M, Yamamoto K, Miyamoto K, Yuasa H, Masuda N, Omotehara T, Tsuruta H, Yokoyama T, Hoshi KH (2018) Ultrastructural and immunohistochemical study on the lamina propria cells beneath Paneth cells in the rat ileum. Anat Rec 301:1074–1085
Mathan M, Hermos JA, Trier JS (1972) Structural features of the epithelia-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol 52:577–588
Mowat AM, Agace WW (2014) Regional specialization within the intestinal immune system. Nat Rev Immunol 14:667–685
Nakanishi S, Mantani Y, Haruta T, Yokoyama T, Hoshi N (2020) Three-dimensional analysis of neural connectivity with cells in rat ileal mucosa by serial block-face scanning electron microscopy. J Vet Med Sci 82:990–999
Nguyen HB, Thai TQ, Saitoh S, Wu B, Saitoh Y, Shimo S, Fujitani H, Otobe H, Ohno N (2016) Conductive resins improve charging and resolution of acquired images in electron microscopic volume imaging. Sci Rep 6:23721
Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC (2005) CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254–258
Ogawa T, Koerten HK, Daems WTH (1978) Peroxidatic activity in monocytes and tissue macrophages of mice. Cell Tissue Res 188:361–373
Ohno N, Katoh M, Saitoh Y, Saitoh S, Ohno S (2015) Three-dimensional volume imaging with electron microscopy toward connectome. Microscopy 64:17–26
Pfeifer CR, Shomorony A, Aronova MA, Zhang G, Cai T, Xu H, Notkins AL, Leapman RD (2015) Quantitative analysis of mouse pancreatic islet architecture by serial block-face SEM. J Struct Biol 189:44–52
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361–367
Schridde A, Bain CC, Mayer JU, Montgomery J, Pollet E, Denecke B, Milling SWF, Jenkins SJ, Dalod M, Henri S, Malissen B, Pabst O, Mcl MA (2017) Tissue-specific differentiation of colonic macrophages requires TGFβ receptor-mediated signaling. Mucosal Immunol 10:1387–1399
Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O (2009) Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206(3101):3114
Shami GJ, Cheng D, Huynh M, Vreuls C, Wisse E, Braet F (2016) 3-D EM exploration of the hepatic microarchitecture – lessons learned from large-volume in situ serial sectioning. Sci Rep 6:36744
Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle‐Murray T, Strangward P, Ridley AJL, Wang P, Tamoutounour S, Allen JE, Konkel JK, Grainger JR (2018) Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J Exp Med 215
Sehgal A, Donaldson DS, Pridans C, Sauter KA, Hume DA, Mabbott NA (2018) The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nat Commun 9:1272
Takahashi-Iwanaga H, Iwanaga T, Isayama H (1999) Porosity of the epithelial basement membrane as an indicator of macrophage-enterocyte interaction in the intestinal mucosa. Arch Histol Cytol 62:471–481
Takaki T, Ohno N, Saitoh S, Nagai M, Joh K (2019) Podocyte penetration of the glomerular basement membrane to contact on the mesangial cell at the lesion of mesangial interposition in lupus nephritis: a three-dimensional analysis by serial block-face scanning electron microscopy. Clin Exp Nephrol 23:773–781
Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211:1533–1549
Van Rees EP, Dijkstra CD, Van Der Ende MB, Janse EM, Sminia T (1988) The ontogenetic development of macrophage subpopulations and Ia-positive non-lymphoid cells in gut-associated lymphoid tissue of the rat. Immunology 63:79–85
Vihinen H, Belevich I, Jokitalo E (2013) Three dimensional electron microscopy of cellular organelles by serial block face SEM and ET. Microsc Anal 27:7–10
Williams L, Jarai G, Smith A, Finan P (2002) IL-10 expression profiling in human monocytes. J Leukoc Biol 72:800–809
Zhang Q, Lee WCA, Paul DL, Ginty DD (2019) Multiplexed peroxidase-based electron microscopy labeling enables simultaneous visualization of multiple cell types. Nat Neurosci 22:828–839
Acknowledgements
The Ketjen black used to prevent charging of samples was kindly provided by Dr. Ohno.
Funding
This study was supported by the Japan Society for the Promotion of Science (grant numbers: 16K18813 and 20K15902).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee (permission numbers: 25-06-01 and 30-05-01). All procedures performed in studies involving animals were in accordance with the ethical standards of the institution (the Kobe University Animal Experimentation Regulations) or practice at which the studies were conducted.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mantani, Y., Haruta, T., Nakanishi, S. et al. Ultrastructural and phenotypical diversity of macrophages in the rat ileal mucosa. Cell Tissue Res 385, 697–711 (2021). https://doi.org/10.1007/s00441-021-03457-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03457-0