Abstract
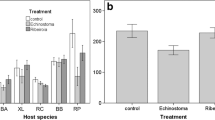
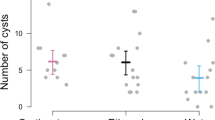
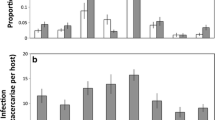
Along with immune defences, many animals exhibit effective anti-parasite behaviours such as parasite avoidance and removal that influence their susceptibility to infection. Host ecology and life history influence investment into comparatively fixed defences such as innate immunity but may affect the strength of anti-parasite behaviours as well. We investigated activity levels in five different species of larval amphibian with varying life histories and ecology in control, novel food stimulus, and trematode parasite (Echinoparyphium sp.) threat conditions. There was a significant interaction of species and treatment given that American toad (Bufo americanus), wood frog (Lithobates sylvaticus), and bullfrog (Lithobates catesbeianus) tadpoles generally increased their activity when parasite infectious stages were present while grey tree frogs (Hyla versicolor) and northern leopard frogs (Lithobates pipiens) did not, even though activity was negatively related to infection. In addition, there was considerable variation among species in their susceptibility to parasitism, with infection prevalence ranging from 17 % in bullfrog tadpoles to 70 % in wood frogs. However, amphibian life history (larval and adult traits) was not related to parasitism or level of anti-parasite behaviour at the species level. Consequently, we suggest that future investigations include more species with a range of life history traits and also consider host ecology, particularly if conspicuous anti-parasite behaviours are more likely in amphibian species that experience a relatively low risk of predation.


Similar content being viewed by others
References
AmphibiaWeb (2013) Information on amphibian biology and conservation. Berkeley, CA. URL http://amphibiaweb.org/. Accessed 25 October 2013
Baker RL, Smith BP (1997) Conflict between antipredator and antiparasite behaviour in larval damselflies. Oecologia 109:622–628
Conant R, Collins JT (1998) A field guide to the reptiles and amphibians of Eastern/Central North America, 3rd edn. Houghton Mifflin, New York
Daly EW, Johnson PTJ (2011) Beyond immunity: quantifying the effects of host anti-parasite behaviour on parasite transmission. Oecologia 165:1043–1050
Edwards DB (2012) Immune investment is explained by sexual selection and pace-of-life, but not longevity in parrots (Psittaciformes). PLoS One 7(12):e53066
Fried B (2001) Biology of echinostomes except Echinostoma. Adv Parasitol 49:163–210
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Grear DA, Luong LT, Hudson PJ (in press) Network transmission inference: host behavior and parasite life-cycle make social networks meaningful in disease ecology. Ecol Appl. doi:10.1890/13-0907.1
Harding JH (2006) Amphibians and Reptiles of the Great Lakes region. The University of Michigan Press, Ann Arbor
Hart BL (1990) Behavioural adaptations to pathogens and parasites: five strategies. Neurosci Biobehav Rev 14:273–294
Hart BL (1997) Behavioural defence. In: Clayton DH, Moore J (eds) Host–parasite evolution: general principle and avian models. Oxford University Press, Oxford, pp 59–77
Hossie TJ, Ferland-Raymond B, Burness G, Murray DL (2010) Morphological and behavioural responses of frog tadpoles to perceived predation risk: a possible role for corticosterone mediation? Ecoscience 17:100–108
Johnson PTJ, McKenzie VJ (2008) Effects of environmental change on helminth infections in amphibians: exploring the emergence of Ribeiroia and Echinostoma infections in North America. In: Fried B, Toledo R (eds) The biology of Echinostomes. Springer, New York, pp 249–280
Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB (2012) Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol Lett 15:235–242
Kats LB, Petranka JW, Sih A (1988) Antipredator defenses and the persistence of amphibian larvae with fish. Ecology 69:1865–1870
Klasing KC, Leshchinsky T (1999) Functions, costs, and benefits of the immune system during development and growth. In: Adams N, Slotow R (eds) Proceedings of the 22nd International Ornithological Congress. Durban University of Natal Press, Durban, pp 2817–2835
Koprivnikar J, Forbes MR, Baker RL (2006) On the efficiency of antiparasite behaviour: a case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Can J Zool 84:1623–1629
Koprivnikar J, Gibson CH, Redfern JC (2012a) Infectious personalities: behavioural syndromes and disease risk in larval amphibians. Proc R Soc Lond B 279:1544–1550
Koprivnikar J, Marcogliese DJ, Rohr JR, Orlofske SA, Raffel TR, Johnson PTJ (2012b) Macroparasite infections of amphibians: what can they tell us? Ecohealth 9:342–360
Lannoo M (2005) Amphibian declines: the conservation status of United States species. University of California Press, Berkeley
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
Martin LB, Hasselquist D, Wikelski M (2006) Investment in immune defense is linked to pace of life in house sparrows. Oecologia 147:565–575
McDiarmid RW, Altig R (1999) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago
Moore J (2002) Parasites and the behaviour of animals. Oxford University Press, Oxford
Preston WB (1982) The amphibians and reptiles of manitoba. Hignell, Winnipeg
Relyea RA (2001a) Morphological and behavioural plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2001b) The relationship between predation risk and antipredator responses in larval anurans. Ecology 82:541–554
Relyea RA (2002) Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol Monogr 72:77–93
Rohr JR, Raffel TR, Hall CA (2010) Developmental variation in resistance and tolerance in a multi-host–parasite system. Funct Ecol 24:1110–1121
Schotthoefer AM, Cole RA, Beasley VR (2003) Relationship of tadpole stage to location of echinostome cercariae encystment and the consequences for tadpole survival. J Parasitol 89:475–482
Skelly DK, Bolden SR, Holland MP, Freidenburg LK, Friedenfelds NA, Malcolm TR (2006) Urbanization and disease in amphibians. In: Collinge SK, Ray C (eds) Disease ecology: community structure and pathogen dynamics. Oxford University Press, Cary, pp 153–167
Smith GR, Boyd A, Dayer CB, Ogle ME, Terlecky AJ (2009) Responses of grey treefrog and American toad tadpoles to the presence of cues from multiple predators. Herpetol J 19:79–83
Stahlschmidt ZR, Rollinson N, Acker M, Adamo SA (2013) Are all eggs created equal? Food availability and the fitness trade-off between reproduction and immunity. Funct Ecol 27:800–806
Stebbins RC (1951) Amphibians of western North America. University of California Press, Berkeley
Szuroczki D, Richardson JML (2011) Palatability of the larvae of three species of Lithobates. Herpetologica 67:213–221
Szuroczki D, Richardson JML (2012) The behavioral response of larval amphibians (Ranidae) to threats from predators and parasites. PLoS One 7(11):e49592
Taylor CN, Oseen KL, Wassersug RJ (2004) On the behavioural response of Rana and Bufo tadpoles to Echinostomatoid cercariae: implications to synergistic factors influencing trematode infections in anurans. Can J Zool 82:701–706
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:S9–S22
Acknowledgments
We thank D. Szuroczki and B. Pickering for aid in obtaining amphibian eggs and larvae as well as the anonymous reviewer of an earlier manuscript for helpful comments. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant no. 386691-2010) and the Brandon University Research Council to J. K., as well as NSERC Undergraduate Student Research Awards to J. C. R. and H. L. M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Marcogliese.
Rights and permissions
About this article
Cite this article
Koprivnikar, J., Redfern, J.C. & Mazier, H.L. Variation in anti-parasite behaviour and infection among larval amphibian species. Oecologia 174, 1179–1185 (2014). https://doi.org/10.1007/s00442-013-2857-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2857-7