Abstract
Background:
To evaluate the use of esophageal stents for temporary sealing of acquired benign tracheoesophageal fistulas developed in critically ill, ventilated patients.
Methods:
This is a retrospective analysis (1992–2003) of the data of 12 mechanically ventilated patients — six of them after major or multiple trauma — being intubated for a median of 30 days before they develop an acquired benign tracheoesophageal fistula. Five of them were in sepsis. Two types of stents were used: the Wilson-Cook esophageal balloon plastic stent in the first four cases and the Ultraflex covered self-expandable stent in the remaining eight. The total procedure was performed at bedside in the intensive care unit, with no special need for supplementary anesthesia or fluoroscopic control.
Results:
Stent implantation was technically successful in all patients and fistula occlusion was achieved in every case. There was no stent migration and fistulas remained sealed until death or upon decision for removal. Nine patients died between 5 days and 2 months after stent placement, as a result of their diseases. Three patients were referred for fistula surgical repair 33, 36, and 43 days after stent placement. Before surgery the stents were easily removed under direct vision.
Conclusion:
Temporary closure of an acquired tracheoesophageal fistula developed in critically ill ventilated patients is an easy, bedside-applicable, safe, and effective palliative procedure, with no complications or mortality.
Similar content being viewed by others
Benign-acquired tracheoesophageal fistula (TEF) is an infrequent complication of prolonged ventilation and tracheostomy [4, 35]. Although the widespread use of high-volume low-pressure cuffs has reduced the incidence of cuff-related TEF [11, 18], long-term intubation still accounts for the majority of cases, because of either overinflation of the cuff or placement of small tracheostomy tubes necessitating overinflation of their cuffs to provide airway sealing [7]. Another cause, recently recognized, is occult laceration injuries of the mucosal surface and involving, but not through, the underlying mucosal layer of the posterior wall of the trachea; these are assumed to be caused by poor stabilization of the guidewire and guiding catheter during percutaneous tracheostomy [21, 31]. Associated risk factors involved include excessive motion of the tube, infections, hypotension-tissue ischemia, steroids, diabetes, head position, and the presence of a nasogastric tube [16, 22].
In ventilator-dependent patients, once TEF forms, it presents difficult management problems, since a substantial portion of the delivered tidal volume may be lost through the tracheal defect, resulting in a dramatic decrease of alveolar ventilation with CO2 retention [22].
Although placement of the tracheostomy tube cuff distal to the fistula, to minimize tracheobronchial soilage, and induction of a percutaneous endoscopic gastrostomy to diminish reflux and to avoid nasogastric tube irritation are the immediate measures of conservative treatment [16, 26], operative closure of TEFs is almost always necessary because spontaneous closure is so rare [4, 6, 7, 12]. However, surgical repair should be postponed until the patient is in a fully stabilized condition and weaned from mechanical ventilation, since positive pressure ventilation after tracheal repair carries an increased risk of anastomotic dehiscence and restenosis [26]. Thus, temporary closure of the defect by means of a minimally invasive procedure would be the most desired method for TEF preoperative management.
Endoscopic placement of esophageal stents is a well-established, safe, easy, and effective method for the palliative treatment of esophago-respiratory fistulas due to malignancy. However, their use for benign conditions was considered to be relatively contraindicated, because of concerns about an increased risk of migration, perforation, or bleeding, but mainly because of the absence of information regarding long-term sequelae, and lack of experience regarding their removal [9, 10, 15].
In the present study we report our experience of the application of such a procedure in 12 critically ill patients, who developed an acquired TEF during their hospitalization in the intensive care unit (ICU).
Materials and mathods
Patients
Since 1992, 12 mechanically ventilated patients with TEF have been referred to us from our hospital ICUs. Their demographic characteristics are presented in Table 1.
All patients had been subjected to tracheostomy a median of 16 days (range, 5–62 days) before diagnosis of TEF, and the overall median intubation time was 30 days (range, 15–80 days). Five patients had a conventional surgical tracheostomy and a nasogastric tube, and the other seven, a percutaneous operated one; three patients had a nasogastric tube, and four had been subjected to percutaneous endoscopic gastrostomy at the time of the tracheostomy procedure. The clinical suspicion of TEF arose from a sudden decrease of alveolar ventilation with CO2 retention and the need for overinflation of the cuff; additionally, enteral feeding solution reflux and leak from the tracheal stoma was recognized in three cases. A total of five patients were in sepsis: three of six trauma patients and the two intubated after postoperative complications.
Endoscopic diagnosis
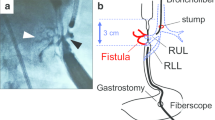
Diagnosis of TEF was confirmed by endoscopy. A 10-mm endoscope was first inserted under direct visualization of the esophageal opening by means of a laryngoscope and advanced for upper GI tract inspection. During withdrawal a careful inspection of the upper esophagus under continuous air inflation revealed at least the fistula’s opening, and in the cases of a huge opening, the cuff of the tracheostomy tube protruded into the esophageal lumen (Fig. 1). At the same time, illumination under or just below the tracheostomy stoma was prominent. The exact characteristics of the TEF, i.e., its size and the distance of its proximal end from the upper esophageal sphincter, as well as the presence of esophageal mucosal erosions, were carefully recorded (Table 2).
Endoscopic intervention for stent placement
During the same session a percutaneous endoscopic gastrostomy was then performed for nasogastric tube relief, in the eight cases in which it was still in place.
Since all our patients had to remain under mechanical ventilation for a long period and had, additionally, at least one more contraindication for early surgical repair, i.e., sepsis, poor prognosis, underlying diseases, or advanced age, it was decided to temporarily manage the condition with an esophageal stent.
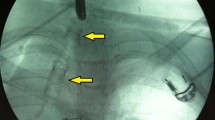
Two types of stents were used: the Wilson-Cook (William Cook Europe, Copenhagen, Denmark) esophageal balloon plastic stent in the first four cases and the Ultraflex (Microvasive, Boston Scientific, Natick, MA) covered self-expandable stent in the remaining eight. There was no special need for supplementary anesthesia, the procedure was performed at bedside in the ICU and fluoroscopic control was not used in any case. However, careful measurements were made to ensure proper positioning of the prosthesis. After the procedure, the location of the stent was confirmed, at bedside, by a chest x-ray film.
The Wilson-Cook stent was loaded on its introducer and slipped down with a pusher tube over a guide wire. After insertion, the tracheostomy tube cuff was temporary deflated, the balloon of the stent was inflated with the appropriate volume of air, and the high-volume low-pressure cuff of the tracheal tube was again inflated, thus securing the stent in place. In the first patient the proximal conical end of the stent was additionally secured in place by two silk threads sewed onto it and externalized through the patient’s nostril and sealed with tape. However, we very early recognized that this measure was unnecessary and removed it, since the stent’s balloon and tracheostomy tube’s cuff contact prevented proximal migration and the stent’s proximal conical end prevented distal migration.
The Ultraflex stent, commercially preloaded and compressed on a polypropylene tube, was inserted over the guidewire and expanded after careful measurement of the distances for correct positioning. The upper end (uncovered) of the stent was generally positioned 1–2 cm over the upper esophageal sphincter within the cricopharyngeal muscle, while the use of the proximally released type of stent helped distal migration avoidance during its expansion, because of 20% shortening of the stent.
After insertion, the correct position was confirmed by a simple endoscopic inspection of the stent’s upper part (conical end or uncovered part, respectively) within the hypo-pharynx, and indirectly by the improvement in the patient’s ventilatory parameters. No other diagnostic test was considered necessary. However, fistula occlusion was confirmed, indirectly, during tracheal tube removal for routine change and, directly, in the case of bronchoscopy for respiratory lavage.
Results
Stent implantation was technically successful in all patients and fistula occlusion was also achieved in every case. Patients were followed up until their death or until stent removal for the surgical repair of their tracheas. Nine patients died between 5 days and 2 months after stent placement: five from sepsis, two from respiratory insufficiency, one from heart failure, and one after persistent fever of unknown etiology. There was no stent migration and the fistula remained sealed, as routine bronchoscopies revealed.
The remaining three patients were as follows: a 21-year-old multiple trauma patient (weaned from ventilator 33 days later) and two spinal cord (A7 and T8) injury patients (23 and 48 years old), whose respiratory status improved and needed only ventilatory support 43 and 36 days, respectively, after stent placement.
These three patients were referred for TEF surgical repair. Before surgery the stents — two Wilson-Cook and one Ultraflex stent — were easily removed by simply grasping their proximal end with a pair of retrieval forceps under direct vision.
Discussion
There are multiple reports in the literature outlining the therapeutic efficacy of esophageal stents in treating esophago-respiratory fistulas in patients with malignancy [20, 24, 25, 33]. However, understandable hesitation exists about applying these devices in patients with benign diseases, because of concerns regarding short-term complications and the absence of information regarding long-term sequelae.
Our study group consists of 12 critically ill patients who developed an acquired benign TEF as a complication of long-term mechanical ventilation through a tracheostomy tube. Eight of them were trauma patients and the remaining four had a medical pathology requiring mechanical ventilation. Five out of twelve patients suffered severe sepsis.
However, the severity of their illness, as well as the absolute need for mechanical ventilatory support, made surgical repair of TEF completely contraindicated [26]; thus priority had to be given to a minimally invasive procedure, targeting trachea sealing, in order to stop air leakage and thus to improve reduced alveolar ventilation, independently of the possibility of complication occurring.
The main initial problem was to find a method of securing the tube to prevent migration. In the first patient, where a Wilson-Cook balloon stent was inserted, we tied two threads at the conical (proximal) part of the tube and brought them out through the nostril and secured them with tape on the patients’ auricle. However, it almost immediately became clear that the conical part of the tube, in conjunction with the cuff, was all that was needed for the stent to remain in place. When we turned to the Ultraflex self-expanding covered stent we expected migration, since the reported migration from benign cases was as high as 24% [10], probably related to a smaller mucosal surface area with less inward force anchoring the device. Fortunately, in our eight cases, the outward pressure of the tracheostomy tube’s cuff as well as the constriction of the proximal uncovered part of the stent by the upper esophageal sphincter kept the stent in place for up to 2 months. An additional parameter could be the fact that patients were fully anesthetized, and thus head movements were avoided.
A serious problem was the existence of a specification against the other benign esophago-respiratory fistulas referred to in the literature: These TEFs, after long-term mechanical ventilation through a tracheostomy tube, are high up in the upper part of trachea and the cervical esophagus, respectively, just opposite or 1–2 cm below the tracheal stoma. This high position is considered a relative contraindication for stent placement, because of concerns about the increased risk of migration of the prosthesis into the hypopharynx, i.e., proximally and, most importantly, an intolerable sensation of a foreign body [13, 28]. Regarding the latter contraindication, patients presented had no pharyngeal refluxes because of deep anesthesia; regarding the former, because of the high position of the stent in the esophagus its upper part was already in the hypopharyngeal level and it was thus easily inspected by the use of a laryngoscope. This is also why removal of the stent was so easy in the presented cases, by simply grasping it with two grasping forceps and under direct vision. In any case, although worldwide experience concerning pharyngeal stents even for malignancy is still limited, current thought is that the presence of a lesion within 2 cm of the cricopharyngeal muscle should no longer be considered a contraindication for stent placement, particularly of one that is soft and flexible [9].
Another point to be discussed is the type of stent used. In four cases, the Wilson-Cook plastic balloon stent was used. This stent may ensure adequate anchorage in the gap, thus achieving mechanical sealing of the fistula. However, its disadvantage is that it is rather hard, with no flexibility and with a larger external diameter in relation to a metal expandable stent of the same inner diameter [1]. In any case, at the time we used the plastic stents, this type was the only one commercially available. The remaining eight cases were treated with covered self-expanding metal stents, namely the Ultraflex stent. This is made from a knitted Nitinol mesh and has the weakest radial force but greater flexibility, thus it may be the best for tortuous upper-third lesions [13]. For the present cases we choose the proximally released stent; this function, in conjunction with its advantage of rapid expansion to the full diameter when released, enabled us to secure the proximal end of the stent in exactly the correct position, proximally to the fistula, with no need for fluoroscopy but only under endoscopic vision, despite the discouraging report Profili et al. [23]. Retrospectively, comparing the two types of stents, we suggest the Ultraflex one, since it fits better to the upper esophagus configuration, its smooth surface at each edge making it atraumatic when positioned in the hypopharynx, despite continuous soliciting during swallowing and, finally, in the case of awake patients (personal unpublished data) gives a tolerable foreign body sensation.
The uncovered ends of the Ultraflex stent frequently tend to become incorporated into the mucosa and submucosal layers of the esophageal wall, which leads to both difficulties in removal, in cases of benign lesions, and further on, to pressure necrosis and hemorrhage or fistulization [2, 10, 15]. This process, although decreasing the tendency for migration, leads to serious problems in benign cases where removal is desired. In the patient whose condition improved and who was referred to be operated on for trachea reconstruction, we experienced no problem during stent removal 36 days postimplantation. This is in agreement with the present thought that expandable, metallic stents may ultimately serve as a routine option for the treatment of benign lesions, if the stents can be safely removed within 3 months or before the end of the evolutionary process of epithelial hyperplasia penetrating their uncovered ends [9, 15, 29].
Regarding the sealing rate, we achieved a 100% sealing with both types of stent. For the Wilson-Cook stent it is somewhat obvious, since it is designed for such a use, i.e., the foamed, air-filled balloon to invade and seal the fistula. For the Ultraflex, although it is not specially designed for such use, its rapid expansion caused tight fixation to the esophageal wall and sealing of the TEF at a rate of 73% to 100%, according to different series [1, 12, 19, 27, 30], referring both to malignant and benign fistulas.
Additionally, no patient experienced hemorrhage during the period the stent was in place, and none of the three patients who survived and were operated on for trachea reconstruction were referred for esophageal stenosis due to esophageal mucosal damage.
The final point to be discussed is alternative treatment options, i.e., tracheobroncheal stenting [8, 14] or parallel stenting [5, 8, 32] or fibrin sealant application [3, 34]. In respect to the latter, although it is an attractive method with good results throughout the digestive tract, it prerequires healthy fistula edges, no local infection, and, of course, a split or a small opening. On the other hand, the application of tracheal stent, although an excellent alternative to esophageal, in experienced hands, is fully contraindicated in patients eligible for trachea surgical repair, since stenting or plugging of the tracheoesophageal hole results in a giant TEF, due to continued pressure in this region [16].
In conclusion, the results of the present study in patients suffering TEF due to prolonged ventilation demonstrate that stent placement in the cervical esophagus is an easy, bedside-applicable, safe, and effective palliative procedure, with no complications or mortality. We thus recommend the Ultraflex self-expandable covered stent for temporary sealing of TEFs in any patient whose general condition and the need for mechanical ventilation contraindicate surgical tracheal reconstruction, since stent placement allows the possibility of removal and surgical reintervention when the patient’s condition improves.
References
JM Abadal A Echenagusia G Simo F Camunez (2001) ArticleTitleTreatment of malignant esophagorespiratory fistulas with covered stents Abdom Imaging 26 565–569 Occurrence Handle10.1007/s002610000193 Occurrence Handle11911165
R Ackroyd DI Watson PG Devitt GG Jamieson (2001) ArticleTitleExpandable metallic stents should not be used in the treatment of benign esophageal strictures J Gastroenterol Hepatol 16 484–487 Occurrence Handle10.1046/j.1440-1746.2001.02367.x Occurrence Handle11354292
I Benko TF Molnar OP Horvath (1997) ArticleTitleA case of fibrin sealant application for closing benign trachea-esophageal fistula (TEF) Acta Chir Hung 36 25–86 Occurrence Handle9408274
A Cherveniakov C Tzekov GE Grigorov P Cherveniakov (1996) ArticleTitleAcquired benign esophago-airway fistulas Eur J Cardiothorac Surg 10 713–716 Occurrence Handle10.1016/S1010-7940(96)80329-0 Occurrence Handle8905271
HG Colt B Meric JF Dumon (1992) ArticleTitleDouble stents for carcinoma of the esophagus invading the tracheo-bronchial tree Gastrointest Endosc 38 485–489 Occurrence Handle1380932
L Couraud D Bercovici L Zanotti P Clerc JF Velly J Dubrez (1989) ArticleTitleTreatment of esophagotracheal fistula following intensive care. An experience of 17 cases Ann Chir 43 677–681 Occurrence Handle2686515
P Dartevelle P Macchiarini (1996) ArticleTitleManagement of acquired tracheoesophageal fistula Chest Surg Clin N Am 6 819–836 Occurrence Handle8934011
L Freitag E Tekolf H Steveling TJ Donovan G Stamatis (1996) ArticleTitleManagement of malignant esophagotracheal fistulas with airway stenting and double stenting Chest 110 1155–1160 Occurrence Handle8915213
GT Gislason PJ Pasricha (1997) ArticleTitleCrossing the upper limit: esophageal stenting in the proximal esophagus Dysphagia 12 84–85 Occurrence Handle9071808
JE Hramiec MA O’Shea RM Quinlan (1998) ArticleTitleExpandable metallic esophageal stents in benign disease: a cause for concern Surg Laparosc Endosc 8 40–43 Occurrence Handle10.1097/00019509-199802000-00010 Occurrence Handle9488569
H LeBrigand B Roy (1966) ArticleTitleFistules tracheo-oesophagiennes après tracheotomies: a propos de 4 observations Mem Acad Chir 92 405–410 Occurrence Handle5941411
JG Lee R Hsu JW Leung (2000) ArticleTitleAre self-expanding metal mesh stents useful in the treatment of benign esophageal stenoses and fistulas? An experience of four cases Am J Gastroenterol 95 1920–1925 Occurrence Handle10.1111/j.1572-0241.2000.02246.x Occurrence Handle10950036
SH Lee (2001) ArticleTitleThe role of oesophageal stenting in the non-surgical management of oesophageal strictures Br J Radiol 74 891–900 Occurrence Handle11675304
DE Low RA Kozarek (1998) ArticleTitleComparison of conventional and wire mesh expandable prostheses and surgical bypass in patients with malignant esophagorespiratory fistulas Ann Thorac Surg 65 919–923 Occurrence Handle10.1016/S0003-4975(98)00081-2 Occurrence Handle9564901
DE Low RA Kozarek (2003) ArticleTitleRemoval of esophageal expandable metal stents: description of technique and review of potential applications Surg Endosc 17 990–996 Occurrence Handle10.1007/s00464-002-8528-0 Occurrence Handle12806523
P Macchiarini JP Verhoye A Chapelier E Fadel P Dartevelle (2000) ArticleTitleEvaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas J Thorac Cardiovasc Surg 119 268–276 Occurrence Handle10.1067/mtc.2000.106039 Occurrence Handle10649202
CH Marty-Ane E Picard O Jonquet H Mary (1995) ArticleTitleMembranous tracheal rupture after endotracheal intubation Ann Thorac Surg 60 1367–1371 Occurrence Handle10.1016/0003-4975(95)00643-Y Occurrence Handle8526628
DJ Mathisen HC Grillo JC Wain (1991) ArticleTitleManagement of acquired nonmalignant tracheoesophageal fistula Ann Thorac Surg 52 759–765 Occurrence Handle1929626
A May C Ell (1998) ArticleTitlePalliative treatment of malignant esophagorespiratory fistulas with Gianturco-Z stents: A prospective clinical trial and review of the literature on covered metal stents Am J Gastroenterol 93 532–535 Occurrence Handle9576443
V Mohan RA Kozarek (2001) ArticleTitlePlacement of conventional and expandable stents for malignant esophageal stenoses Techniques Gastrointest Endosc 3 166–175
S Norwood VL Vallina K Short M Saigusa LG Fernandez JW McLarty (2000) ArticleTitleIncidence of tracheal stenosis and other late complications after percutaneous tracheostomy Ann Surg 232 233–241 Occurrence Handle10.1097/00000658-200008000-00014 Occurrence Handle10903603
DK Payne WM Anderson MD Romero DR Wissing M Fowler (1990) ArticleTitleTracheoesophageal fistula formation in intubated patients. Risk factors and treatment with high-frequency jet ventilation Chest 98 161–164 Occurrence Handle2361384
S Profili GB Meloni CF Feo A Pischedda C Bozzo GC Ginesu GC Canalis (2002) ArticleTitleSelf-expandable metal stents in the management of cervical oesophageal and/or hypopharyngeal strictures Clin Radiol 57 1028–1033 Occurrence Handle10.1053/crad.2002.0988 Occurrence Handle12409115
I Raijman I Siddique J Ajani P Lynch (1998) ArticleTitlePalliation of malignant dysphagia and fistulae with coated expandable metal stents: experience with 101 patients Gastrointest Endosc 48 172–179 Occurrence Handle9717783
FC Ramirez B Dennert ST Zierer RA Sanowski (1997) ArticleTitleEsophageal self-expandable metallic stents–indications, practice, techniques, and complications: results of a national survey Gastrointest Endosc 45 360–364 Occurrence Handle9165315
MF Reed DJ Mathisen (2003) ArticleTitleTracheoesophageal fistula Chest Surg Clin N Am 13 271–289 Occurrence Handle10.1016/S1052-3359(03)00030-9 Occurrence Handle12755313
RR Saxon RE Barton RM Katon PC Lakin HA Timmermans BT Uchida FS Keller J Rosch (1995) ArticleTitleTreatment of malignant esophagorespiratory fistulas with silicone-covered metallic Z stents J Vasc Interv Radiol 6 237–242 Occurrence Handle7540442
A Segalin P Granelli L Bonavina C Siardi L Mazzoleni A Peracchia (1994) ArticleTitleSelf-expanding esophageal prosthesis. Effective palliation for inoperable carcinoma of the cervical esophagus Surg Endosc 8 1343–1345 Occurrence Handle10.1007/BF00188298 Occurrence Handle7530383
HY Song HY Jung SI Park SB Kim DH Lee SG Kang Y Il Min (2002) ArticleTitleCovered retrievable expandable nitinol stents in patients with benign esophageal strictures: initial experience Radiology 217 551–557
F Tomaselli A Maier O Sankin M Woltsche H Pinter FM Smolle-Juttner (2001) ArticleTitleSuccessful endoscopical sealing of malignant esophageotracheal fistulae by using a covered self-expandable stenting system Eur J Cardiothorac Surg 20 734–738 Occurrence Handle10.1016/S1010-7940(01)00867-3 Occurrence Handle11574216
SJ Trottier PB Hazard SA Sakabu JH Levine BR Troop JA Thompson R McNary (1999) ArticleTitlePosterior tracheal wall perforation during percutaneous dilational tracheostomy: an investigation into its mechanism and prevention Chest 115 1383–1389 Occurrence Handle10.1378/chest.115.5.1383 Occurrence Handle10334157
HJ Bongard ParticleVan den H Boot P Baas BG Taal (2002) ArticleTitleThe role of parallel stent insertion in patients with esophagorespiratory fistulas Gastrointest Endosc 55 110–115 Occurrence Handle10.1067/mge.2002.119731 Occurrence Handle11756930
N Weigert H Neuhaus T Rosch W Hoffmann HJ Dittler M Classen (1995) ArticleTitleTreatment of esophagorespiratory fistulas with silicone-coated self-expanding metal stents Gastrointest Endosc 41 490–496 Occurrence Handle7615229
NE Wiseman (1995) ArticleTitleEndoscopic closure of recurrent tracheoesophageal fistula using Tisseel J Pediatr Surg 30 1236–1237 Occurrence Handle10.1016/0022-3468(95)90031-4 Occurrence Handle7472992
M Wolf A Yellin YP Talmi E Segal M Faibel J Kronenberg (2000) ArticleTitleAcquired tracheoesophageal fistula in critically ill patients Ann Otol Rhinol Laryngol 109 731–735 Occurrence Handle10961805
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eleftheriadis, E., Kotzampassi, K. Temporary stenting of acquired benign tracheoesophageal fistulas in critically ill ventilated patients. Surg Endosc 19, 811–815 (2005). https://doi.org/10.1007/s00464-004-9137-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-004-9137-x