Abstract
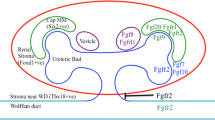
Fibroblast growth factor receptors (Fgfrs) are expressed throughout the developing kidney. Several early studies have shown that exogenous fibroblast growth factors (Fgfs) affect growth and maturation of the metanephric mesenchyme (MM) and ureteric bud (UB). Transgenic mice that over-express a dominant negative receptor isoform develop renal aplasia/severe dysplasia, confirming the importance of Fgfrs in renal development. Furthermore, global deletion of Fgf7, Fgf10, and Fgfr2IIIb (isoform that binds Fgf7 and Fgf10) in mice leads to small kidneys with fewer collecting ducts and nephrons. Deletion of Fgfrl1, a receptor lacking intracellular signaling domains, causes severe renal dysgenesis. Conditional targeting of Fgf8 from the MM interrupts nephron formation. Deletion of Fgfr2 from the UB results in severe ureteric branching and stromal mesenchymal defects, although loss of Frs2α (major signaling adapter for Fgfrs) in the UB causes only mild renal hypoplasia. Deletion of both Fgfr1 and Fgfr2 in the MM results in renal aplasia with defects in MM formation and initial UB elongation and branching. Loss of Fgfr2 in the MM leads to many renal and urinary tract anomalies as well as vesicoureteral reflux. Thus, Fgfr signaling is critical for patterning of virtually all renal lineages at early and later stages of development.



Similar content being viewed by others
References
(2010) North American Pediatric Renal Trials and Collaborative Studies: 2010 Annual Report. Rockville, pp 1–100
Passos-Bueno MR, Wilcox WR, Jabs EW, Sertie AL, Alonso LG, Kitoh H (1999) Clinical spectrum of fibroblast growth factor receptor mutations. Hum Mutat 14:115–125
Cohen MM Jr, Kreiborg S (1993) Visceral anomalies in the Apert syndrome. Am J Med Genet 45:758–760
Sergi C, Stein H, Heep JG, Otto HF (1997) A 19-week-old fetus with craniosynostosis, renal agenesis and gastroschisis: case report and differential diagnosis. Pathol Res Pract 193:579–585, discussion 587-578
Seyedzadeh A, Kompani F, Esmailie E, Samadzadeh S, Farshchi B (2008) High-grade vesicoureteral reflux in Pfeiffer syndrome. Urol J 5:200–202
Tohya T, Miura K, Nagata N (1986) A case of thanatophoric dwarfism with renal hypoplasia. Pediatr Int 28:232–237
Prontera P, Sensi A, Pilu G, Baldi M, Baffico M, Bonasoni R, Calzolari E (2006) FGFR3 mutation in thanatophoric dysplasia type 1 with bilateral cystic renal dysplasia: coincidence or a new association? Genet Couns 17:407–412
Dressler GR (2006) The cellular basis of kidney development. Annu Rev Cell Dev Biol 22:509–529
Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I (2000) Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105:863–873
Guillaume R, Bressan M, Herzlinger D (2009) Paraxial mesoderm contributes stromal cells to the developing kidney. Dev Biol 329:169–175
Powers CJ, McLeskey SW, Wellstein A (2000) Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7:165–197
Gerber SD, Steinberg F, Beyeler M, Villiger PM, Trueb B (2009) The murine Fgfrl1 receptor is essential for the development of the metanephric kidney. Dev Biol 335:106–119
Cancilla B, Ford-Perriss MD, Bertram JF (1999) Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int 56:2025–2039
Fuhrmann V, Kinkl N, Leveillard T, Sahel J, Hicks D (1999) Fibroblast growth factor receptor 4 (FGFR4) is expressed in adult rat and human retinal photoreceptors and neurons. J Mol Neurosci 13:187–197
Korhonen J, Partanen J, Alitalo K (1992) Expression of FGFR-4 mRNA in developing mouse tissues. Int J Dev Biol 36:323–329
Peters KG, Werner S, Chen G, Williams LT (1992) Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development (Cambridge, England) 114:233–243
Orr-Urtreger A, Givol D, Yayon A, Yarden Y, Lonai P (1991) Developmental expression of two murine fibroblast growth factor receptors, flg and bek. Development (Cambridge, England) 113:1419–1434
Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM (2004) Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276:403–415
Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM (2006) Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291:325–339
Dudley AT, Godin RE, Robertson EJ (1999) Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13:1601–1613
Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D (1997) Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol 273:F757–F767
Perantoni AO, Dove LF, Karavanova I (1995) Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci USA 92:4696–4700
Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA (1999) Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99:377–386
Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO (2001) TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development (Cambridge, England) 128:1045–1057
Brennan HC, Nijjar S, Jones EA (1999) The specification and growth factor inducibility of the pronephric glomus in Xenopus laevis. Development (Cambridge, England) 126:5847–5856
Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK (2001) Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev 109:123–135
Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D (1999) FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development (Cambridge, England) 126:547–554
Nguyen HQ, Danilenko DM, Bucay N, DeRose ML, Van GY, Thomason A, Simonet WS (1996) Expression of keratinocyte growth factor in embryonic liver of transgenic mice causes changes in epithelial growth and differentiation resulting in polycystic kidneys and other organ malformations. Oncogene 12:2109–2119
Li Z, Jerebtsova M, Liu XH, Tang P, Ray PE (2006) Novel cystogenic role of basic Fibroblast Growth Factor in developing rodent kidneys. Am J Physiol Renal Physiol 291:F289–296
Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N (2000) FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277:643–649
Sun X, Meyers EN, Lewandoski M, Martin GR (1999) Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev 13:1834–1846
Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR (2005) FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development (Cambridge, England) 132:3847–3857
Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M (2005) Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development (Cambridge, England) 132:3859–3871
Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G (1998) Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J 17:1642–1655
Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM (1996) Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12:390–397
Weinstein M, Xu X, Ohyama K, Deng CX (1998) FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development (Cambridge, England) 125:3615–3623
Revest JM, Spencer-Dene B, Kerr K, De Moerlooze L, Rosewell I, Dickson C (2001) Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol 231:47–62
Steinberg F, Zhuang L, Beyeler M, Kalin RE, Mullis PE, Brandli AW, Trueb B (2010) The FGFRL1 receptor is shed from cell membranes, binds fibroblast growth factors (FGFs), and antagonizes FGF signaling in Xenopus embryos. J Biol Chem 285:2193–2202
Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C (1998) Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development (Cambridge, England) 125:753–765
Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P (1998) Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Nat Acad Sci USA 95:5082–5087
Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J (1994) fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev 8:3032–3044
Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P (1994) Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8:3045–3057
Sims-Lucas S, Argyropoulos C, Kish K, McHugh K, Bertram JF, Quigley R, Bates CM (2009) Three-dimensional imaging reveals ureteric and mesenchymal defects in Fgfr2-mutant kidneys. J Am Soc Nephrol 20:2525–2533
Sims-Lucas S, Cullen-McEwen L, Eswarakumar VP, Hains D, Kish K, Becknell B, Zhang J, Bertram JF, Wang F, Bates CM (2009) Deletion of Frs2alpha from the ureteric epithelium causes renal hypoplasia. Am J Physiol Renal Physiol 297:F1208–1219
Brummer T, Schmitz-Peiffer C, Daly RJ (2010) Docking proteins. FEBS J 277:4356–4369
Eswarakumar VP, Ozcan F, Lew ED, Bae JH, Tome F, Booth CJ, Adams DJ, Lax I, Schlessinger J (2006) Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis. Proc Nat Acad Sci USA 103:18603–18608
Hains D, Sims-Lucas S, Kish K, Saha M, McHugh K, Bates CM (2008) Role of fibroblast growth factor receptor 2 in kidney mesenchyme. Pediatr Res 64:592–598
Wang Y, Tripathi P, Guo Q, Coussens M, Ma L, Chen F (2009) Cre/lox recombination in the lower urinary tract. Genesis 47:409–413
Hains DS, Sims-Lucas S, Carpenter A, Saha M, Murawski I, Kish K, Gupta I, McHugh K, Bates CM (2010) High incidence of vesicoureteral reflux in mice with Fgfr2 deletion in kidney mesenchyma. J Urol 183:2077–2084
Acknowledgements
The author wishes to thank Elsevier and the American Society of Nephrology for permission to reprint some of the figures used in this publication. Some of the work presented was supported by grants from the National Institute of Diabetes and Digestive and Kidney Disease, R01 DK070030 and R01 DK081128 (C. B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bates, C.M. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr Nephrol 26, 1373–1379 (2011). https://doi.org/10.1007/s00467-010-1747-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1747-z