Abstract
Introduction
Preventing CINV is possible when guideline-recommended antiemetics are used. Because oncology nurses play a critical role in risk assessment and management of CINV, a survey of European nurses was conducted to evaluate antiemetic practices, assess awareness of and adherence to current guideline recommendations, and explore barriers to adherence.
Methods
From March 2016 to Feb 2017, 212 oncology nurses in 16 European countries completed a 20-question online survey.
Results
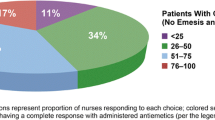
Respondents had 15-year (median) oncology nursing experience, and most (75%) were able to suggest or prescribe antiemetics. Most (80%) worked in the public not-for-profit hospital setting. Guideline awareness was generally low with nurses most familiar with ASCO (46%) and MASCC/ESMO (40%) guidelines; individual institution guidelines were most commonly used (47%). Key discrepancies between reported antiemetic use and guideline recommendations in the highly emetogenic chemotherapy (HEC) setting were underutilization of the recommended NK1RA + 5-HT3RA + steroid combination on day 1 (55%) and high use of 5-HT3RAs (50%) on days 2–5 when a steroid (63% use) should be used. Metoclopramide use was high in both HEC and moderately emetogenic settings, with ~ 30% and ~ 50% reporting use on day 1 and days 2–5, respectively. The most common reported barrier to use of guideline-recommended agents was physician preference (40%). The most common challenges in managing CINV were “controlling nausea/vomiting in the delayed phase” (64%) and “reducing the impact of CINV on patients’ quality-of-life” (61%).
Conclusions
This survey highlights opportunities to improve utilization of guideline-recommended antiemetics, thereby optimizing prevention of CINV and QoL for patients receiving emetogenic chemotherapy.





Similar content being viewed by others
References
Jordan K, Aapro M, Kaasa S, Ripamonti CI, Scotté F, Strasser F, Young A, Bruera E, Herrstedt J, Keefe D, Laird B, Walsh D, Douillard JY, Cervantes A (2018) European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 29(1):36–43
de Boer-Dennert M, de Wit R, Schmitz PIM, Djontono J, v Beurden V, Stoter G, Verweij J (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Schwartzberg L, Harrow B, Lal L et al (2015) Resource utilization for chemotherapy-induced nausea and vomiting events for patients with solid tumors treated with antiemetic regimens. Am Health Drug Benefits 8(5):273–282
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358:2482–2494
Van Laar ES, Desai JM, Jatoi A (2015) Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providers. Support Care Cancer 23:151–157
Navari R, Aapro M (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. NEJM 374:1356–1367
Jordan K, Jahn F, Aapro M (2015) Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol 26(6):1081–1090
Multinational Association of Supportive Care in Cancer (MASCC) Antiemetic Guideline 2013. http://www.mascc.org
Hesketh PJ, Bohlke K, Lyman G, Basch E, Chesney M, Clark-Snow RA, Danso MA, Jordan K, Somerfield MR, Kris MG, American Society of Clinical Oncology (2016) Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 34(4):381–386
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, Antiemesis Version 2, 2015; http://www.nccn.org
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D (2017) Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine Cancer treatment. JAMA 318(2):197–198
ASCO 50th anniversary poll names top 5 advances past 50 years. (Accessed October 24, 2016 at http://www.asco.org/about-asco/press-center/news-releases/asco-50th-anniversary-poll-names-top-5-advances-past-50-years)
Aapro M, Molassiotis A, Dicato M, Peláez I, Rodríguez-Lescure Á, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F, on behalf of the PEER investigators (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis registry (PEER). Ann Oncol 23(8):1986–1992
Gilmore JW, Peacock NW, Gu A, Szabo S, Rammage M, Sharpe J, Haislip ST, Perry T, Boozan TL, Meador K, Cao X, Burke TA (2014) Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract 10(1):68–74
Affronti ML, Schneider SM, Schlundt S et al (2014) Adherence to antiemetic guidelines in patients with malignant glioma: a quality improvement project to translate evidence into practice. Support Care Cancer 22(7):1897–1905
Roeland E, Aapro M, Schwartzberg L (2015) Advances in the Management of Chemotherapy-induced Nausea and Vomiting: new data from recent and ongoing trials. Clinical Roundtable Monograph. Clinical Advances in Hematology & Oncology
Aapro M, Scotte F, Escobar Y, et al (2018) Evaluation of practice patterns for prevention of chemotherapy (CT)-induced nausea and vomiting (CINV) and antiemetic guidelines (GLs) adherence based on real-world prescribing data. European Society of Medical Oncology (ESMO) Annual Meeting (abstract)
Clark-Snow R, Affronti ML, Rittenberg CN (2018) Chemotherapy-induced nausea and vomiting (CINV) and adherence to antiemetic guidelines: results of a survey of oncology nurses. Support Care Cancer 26(2):557–564
Aapro M, Ruffo P, Panteri R, et al (2018) Oncologist perspectives on chemotherapy-induced nausea and vomiting (CINV) management and outcomes: a quantitative market research-based survey. Accepted for publication in Cancer Reports
Vidall C, Fernandez-Ortega P, Cortinovis D et al (2015) Impact and management of chemotherapy/radiotherapy-induced nausea and vomiting and the perceptual gap between oncologists/oncology nurses and patients: a cross-sectional multinational survey. Support Care Cancer 23(11):3297–3305
Navari R, Aapro M (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 374:1356–1367
Sonawane KB, Cheng N, Hansen RA (2018) Serious adverse drug events reported to the FDA: analysis of the FDA adverse event reporting system 2006-2014 database. J Manag Care Spec Pharm 24(7):682–690
Acknowledgements
Cogora, an independent European market access and customer intelligence agency, managed the conduct of the survey and summarized the findings. Editorial and medical writing assistance was provided by Jennifer Vanden Burgt, an independent medical writer, and funded by Helsinn Healthcare, SA, Lugano, Switzerland. The survey was funded by Helsinn Healthcare, SA. The authors are fully responsible for all content and editorial decisions for this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have the following conflicts of interest to disclose:
Dielenseger: Advisory boards: Helsinn, Roche, Shire, Tesaro, BMS, Pfizzer, Janssen Cilag, Bayer Healthcare, IPSEN.
Börjeson: None.
Vidall: None.
Young: Honoraria from Helsinn, Bayer, Leo Pharma; Educational grant from Bayer.
Jahn: Travel support: Helsinn (2014); Consulting or Advisory role: Bristol-Myers Squibb, Chugai, Norgine and Clinigen; Clinical Research Fund by Chugai
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 145 kb)
Rights and permissions
About this article
Cite this article
Dielenseger, P., Börjeson, S., Vidall, C. et al. Evaluation of antiemetic practices for prevention of chemotherapy-induced nausea and vomiting (CINV): results of a European oncology nurse survey. Support Care Cancer 27, 4099–4106 (2019). https://doi.org/10.1007/s00520-019-04697-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04697-1