Abstract
Purpose
Chemotherapy-induced gastrointestinal toxicity is a common adverse event during chemotherapeutic treatment. No uniformly applicable strategies exist to predict, prevent, or treat gastrointestinal toxicity. Thus, a goal of mucositis research is to identify targets for therapeutic interventions and individualized risk prediction. Fibrinogen C domain containing 1 (FIBCD1) is a transmembrane protein expressed in human intestinal epithelial cells with functions in the innate immune system. Previous observations have shown that FIBCD1 ameliorates dextran sulfate sodium (DSS)–induced intestinal inflammation in vivo. We evaluated the effect of FIBCD1 in a murine model of chemotherapy-induced gastrointestinal toxicity and inflammation.
Methods
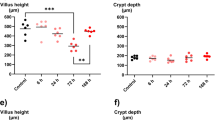
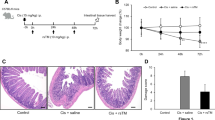
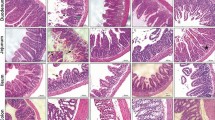
Transgenic (Tg) mice overexpressing FIBCD1 in the intestinal epithelium (Fibcd1Tg) and wild-type (WT) littermates (C57BL/6N) were randomized to receive an intraperitoneal injection of doxorubicin 20 mg/kg or saline and were terminated 2 or 7 days after the injection. Gastrointestinal toxicity was evaluated by weight change, intestinal length, villus height/crypt depth, and histological mucositis score. Expression of inflammatory markers (IL-6, IL-1β, and Tnfα) was measured by quantitative real-time PCR in intestinal tissue samples.
Results
Following doxorubicin treatment, WT mice exhibited an increased weight loss compared with Tg littermates (p < 0.001). No differences between genotypes were seen in mucositis score, intestinal length, villus height/crypt depth, or IL-6, IL-1β, and Tnfα expression.
Conclusion
Our findings suggest that FIBCD1 could ameliorate chemotherapy-induced gastrointestinal toxicity by reducing weight loss; however, the mechanism of this possible protective effect remains to be defined warranting additional investigations.




Similar content being viewed by others
References
Rathe M, Sorensen GL, Wehner PS, Holmskov U, Sangild PT, Schmiegelow K, Muller K, Husby S (2017) Chemotherapeutic treatment reduces circulating levels of surfactant protein-D in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 64(3). https://doi.org/10.1002/pbc.26253
Lund B, Asberg A, Heyman M, Kanerva J, Harila-Saari A, Hasle H, Soderhall S, Jonsson OG, Lydersen S, Schmiegelow K (2011) Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer 56(4):551–559. https://doi.org/10.1002/pbc.22719
Prucker C, Attarbaschi A, Peters C, Dworzak MN, Potschger U, Urban C, Fink FM, Meister B, Schmitt K, Haas OA, Gadner H, Mann G (2009) Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Munster study group. Leukemia 23(7):1264–1269. https://doi.org/10.1038/leu.2009.12
Kuiken NS, Rings EH, Tissing WJ (2015) Risk analysis, diagnosis and management of gastrointestinal mucositis in pediatric cancer patients. Crit Rev Oncol Hematol 94(1):87–97. https://doi.org/10.1016/j.critrevonc.2014.12.009
Christensen MS, Heyman M, Mottonen M, Zeller B, Jonmundsson G, Hasle H (2005) Treatment-related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992-2001. Br J Haematol 131(1):50–58. https://doi.org/10.1111/j.1365-2141.2005.05736.x
Schmiegelow K, Muller K, Mogensen SS, Mogensen PR, Wolthers BO, Stoltze UK, Tuckuviene R, Frandsen T (2017) Non-infectious chemotherapy-associated acute toxicities during childhood acute lymphoblastic leukemia therapy. F1000Research 6:444. https://doi.org/10.12688/f1000research.10768.1
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB, Mucositis Study Section of the Multinational Association for Supportive Care in C, International Society for Oral O (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025. https://doi.org/10.1002/cncr.20162
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4(4):277–284. https://doi.org/10.1038/nrc1318
Al-Dasooqi N, Sonis ST, Bowen JM, Bateman E, Blijlevens N, Gibson RJ, Logan RM, Nair RG, Stringer AM, Yazbeck R, Elad S, Lalla RV (2013) Emerging evidence on the pathobiology of mucositis. Support Care Cancer 21(7):2075–2083. https://doi.org/10.1007/s00520-013-1810-y
Villa A, Sonis ST (2015) Mucositis: pathobiology and management. Curr Opin Oncol 27(3):159–164. https://doi.org/10.1097/cco.0000000000000180
Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, Parisi G, Calvetti L, Sonis ST (2017) New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol 8:354. https://doi.org/10.3389/fphar.2017.00354
Rathe M, De Pietri S, Wehner PS, Frandsen TL, Grell K, Schmiegelow K, Sangild PT, Husby S, Muller K (2019) Bovine colostrum against chemotherapy-induced gastrointestinal toxicity in children with acute lymphoblastic leukemia: a randomized, double-blind, placebo-controlled trial. JPEN J Parenter Enteral Nutr 44:337–347. https://doi.org/10.1002/jpen.1528
von Huth S, Moeller JB, Schlosser A, Marcussen N, Nielsen O, Nielsen V, Sorensen GL, Holmskov U (2018) Immunohistochemical localization of fibrinogen C domain containing 1 on epithelial and mucosal surfaces in human tissues. J Histochem Cytochem 66(2):85–97
Moeller JB, Leonardi I, Schlosser A, Flamar AL, Bessman NJ, Putzel GG, Thomsen T, Hammond M, Jepsen CS, Skjodt K, Fuchtbauer EM, Farber DL, Sorensen GL, Iliev ID, Holmskov U, Artis D (2019) Modulation of the fungal mycobiome is regulated by the chitin-binding receptor FIBCD1. J Exp Med 216(12):2689–2700. https://doi.org/10.1084/jem.20182244
Thomsen T, Schlosser A, Holmskov U, Sorensen GL (2011) Ficolins and FIBCD1: Soluble and membrane bound pattern recognition molecules with acetyl group selectivity. Mol Immunol 48:369–381
Shrive AK, Moeller JB, Burns I, Paterson JM, Shaw AJ, Schlosser A, Sorensen GL, Greenhough TJ, Holmskov U (2014) Crystal structure of the tetrameric fibrinogen-like recognition domain of fibrinogen C domain containing 1 (FIBCD1) protein. J Biol Chem 289(5):2880–2887. https://doi.org/10.1074/jbc.M113.520577
Jepsen CS, Dubey LK, Colmorten KB, Moeller JB, Hammond MA, Nielsen O, Schlosser A, Templeton SP, Sorensen GL, Holmskov U (2018) FIBCD1 binds Aspergillus fumigatus and regulates lung epithelial response to cell wall components. Front Immunol 9:1967. https://doi.org/10.3389/fimmu.2018.01967
Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS (2010) Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7(6):447–449. https://doi.org/10.1038/nmeth.1455
Arioli V, Rossi E (1970) Errors related to different techniques of intraperitoneal injection in mice. Appl Microbiol 19(4):704–705
Steward JP, Ornellas EP, Beernink KD, Northway WH (1968) Errors in the technique of intraperitoneal injection of mice. Appl Microbiol 16(9):1418–1419
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN (1970) Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101(4):478–483. https://doi.org/10.1001/archsurg.1970.01340280030009
Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ (2009) Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol 297(3):G461–G470. https://doi.org/10.1152/ajpgi.90446.2008
Manzano M, Bueno P, Rueda R, Ramirez-Tortosa CL, Prieto PA, Lopez-Pedrosa JM (2007) Intestinal toxicity induced by 5-fluorouracil in pigs: a new preclinical model. Chemotherapy 53(5):344–355. https://doi.org/10.1159/000107724
Duerr CU, Hornef MW (2012) The mammalian intestinal epithelium as integral player in the establishment and maintenance of host-microbial homeostasis. Semin Immunol 24(1):25–35. https://doi.org/10.1016/j.smim.2011.11.002
Drozdowski L, Thomson AB (2006) Intestinal mucosal adaptation. World J Gastroenterol 12(29):4614–4627
Lockhart PB, Sonis ST (1981) Alterations in the oral mucosa caused by chemotherapeutic agents. A histologic study. J Dermatol Surg Oncol 7(12):1019–1025
Nitenberg G, Raynard B (2000) Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol 34(3):137–168
Rigby RJ, Carr J, Orgel K, King SL, Lund PK, Dekaney CM (2016) Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes 7(5):414–423. https://doi.org/10.1080/19490976.2016.1215806
Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM (2009) Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother Pharmacol 63(2):239–251. https://doi.org/10.1007/s00280-008-0732-8
Hamada K, Kakigawa N, Sekine S, Shitara Y, Horie T (2013) Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother Pharmacol 72(4):757–765. https://doi.org/10.1007/s00280-013-2238-2
Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DM (2008) Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 62(1):33–41. https://doi.org/10.1007/s00280-007-0570-0
Kaczmarek A, Brinkman BM, Heyndrickx L, Vandenabeele P, Krysko DV (2012) Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J Pathol 226(4):598–608. https://doi.org/10.1002/path.3009
Sangild PT, Shen RL, Pontoppidan PEL, Rathe M (2017) Animal models of chemotherapy-induced mucositis: translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol:ajpgi.00204.02017. https://doi.org/10.1152/ajpgi.00204.2017
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2012) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet Clin Pathol 41(1):27–31. https://doi.org/10.1111/j.1939-165X.2012.00418.x
Acknowledgments
We gratefully thank the Department of Pathology at Odense University Hospital, Odense, Denmark, for performing the histological analyses.
Funding
This work was supported by a research grant from The Danish Child Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Danish National Committee on Animal Experimentation, J. no. 2017-15-0201-01385). This manuscript was prepared in accordance with the ARRIVE guidelines [34].
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andersen, M.C.E., Johansen, M.W., Nissen, T. et al. FIBCD1 ameliorates weight loss in chemotherapy-induced murine mucositis. Support Care Cancer 29, 2415–2421 (2021). https://doi.org/10.1007/s00520-020-05762-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05762-w