Background
Gastrointestinal stromal tumor (GIST) is the most common type of nonepithelial tumor in the gastrointestinal tract. The gastrointestinal stromal tumor is defined immunohistologically as a c-Kit-positive tumor. For those GISTs that are malignant, the only effective treatment modality has been surgical. Early clinical reports have shown that imatinib mesylate (STI571) produces substantial anticancer activity in patients with metastatic or unresectable GIST.
Methods
Nine Japanese patients who were found clinically and immunohistochemically to have inoperable GISTs were entered into this study. These patients were given 400 mg STI571 orally once daily. We then evaluated the tumor response and the safety of the drug.
Results
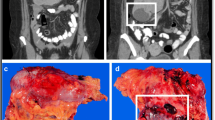
Five of the nine patients achieved partial responses, two had stable disease, and two had progressive disease. The main side effects were skin rash, edema, periorbital edema, diarrhea, nausea, and vomiting. Mild anemia, leukocytopenia, and neutropenia were also noted. No patients required dose reduction or cessation because of adverse events.
Conclusions
This study demonstrates that STI571 might be an active agent against malignant GIST in Japanese patients with manageable toxicities.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sawaki, A., Yamao, K., Nakamura, T. et al. A pilot study of imatinib mesylate (STI571) on gastrointestinal stromal tumors in Japanese patients. J Gastroenterol 39, 329–333 (2004). https://doi.org/10.1007/s00535-003-1298-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00535-003-1298-1